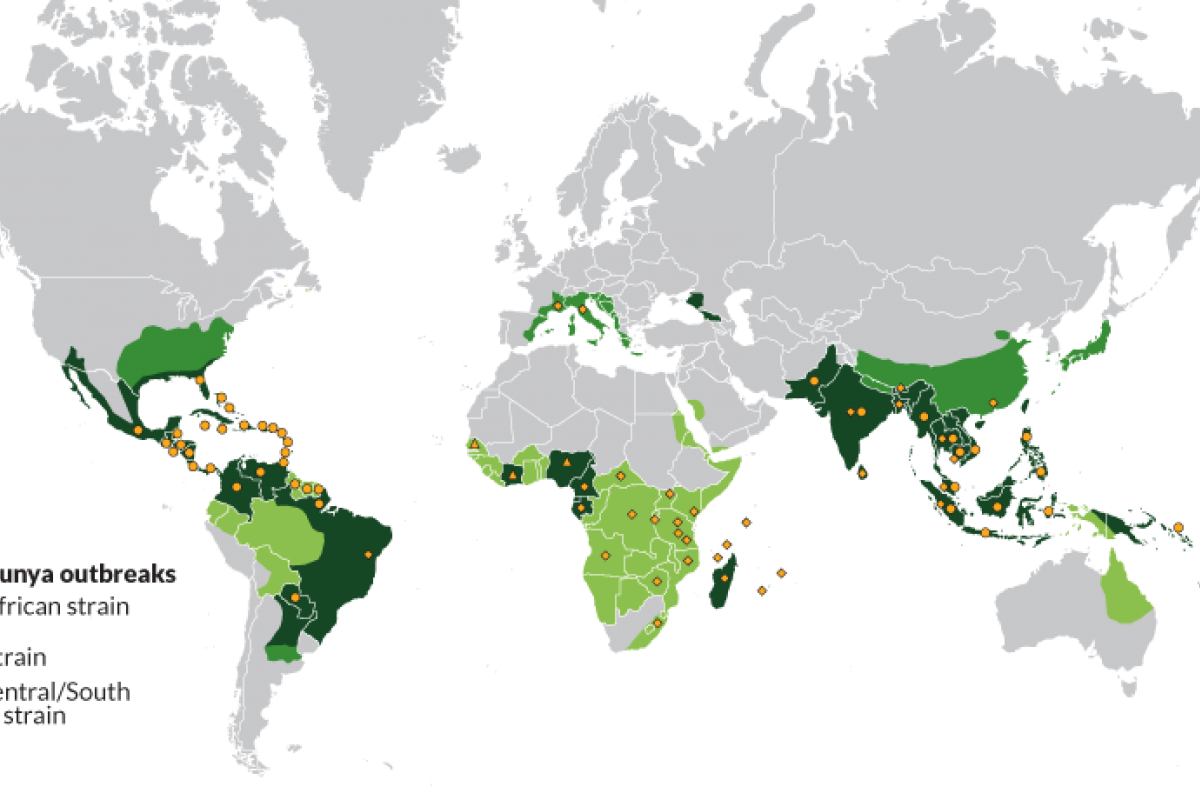
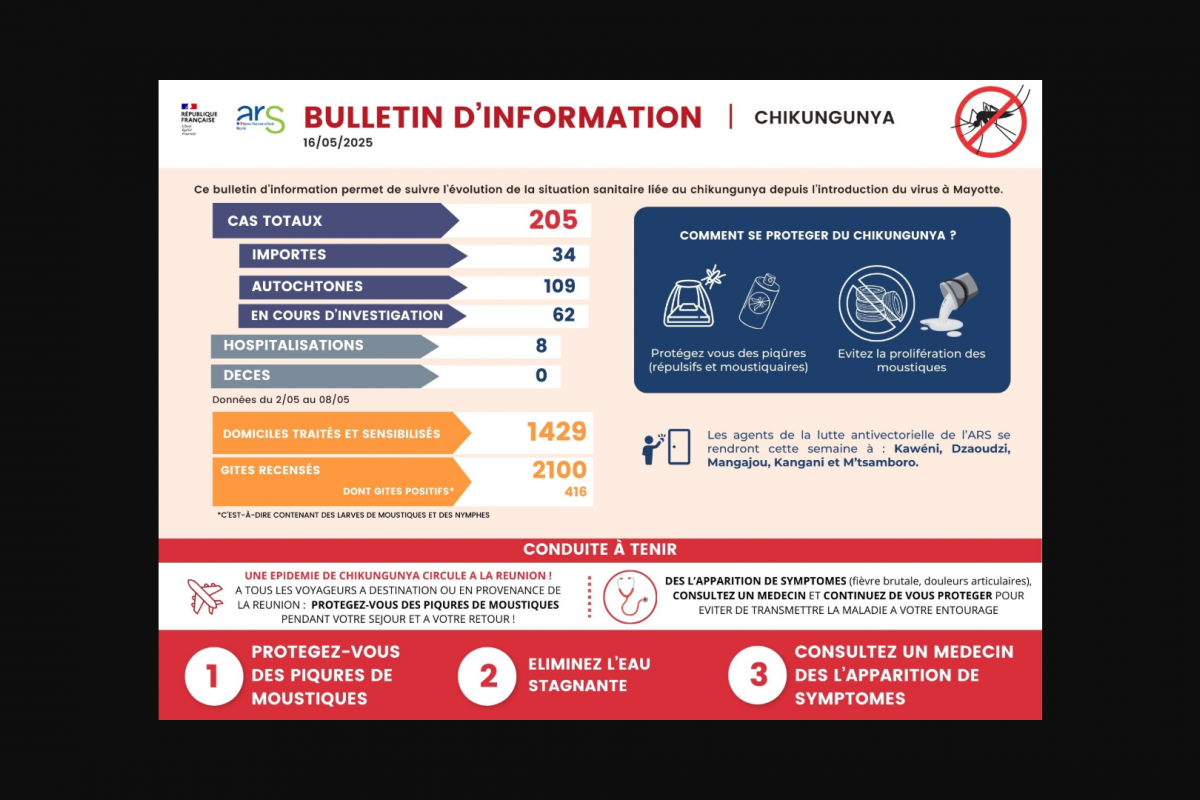
With the mosquito-transmitted Chikungunya virus infecting over 144,000 residents and international travelers throughout the Region of the Americas in 2025, access to an approved vaccine may soon expand.
According to Valneva SE's website, on May 23, 2025, Valneva wrote, 'By 2026, we want to enable access to Valneva's single-shot chikungunya vaccine IXCHIQ® in India and Brazil by enabling local manufacturing and access through technology transfers.'
'In Asia, we signed an exclusive license agreement with the Serum Institute of India, complementing our existing agreement with Instituto Butantan in the Americas.'
IXCHIQ was the first vaccine approved in 2023 to address chikungunya virus infections in adults at increased risk of exposure to the mosquito-transmitted disease. This vaccine is currently available in the United States at clinics and pharmacies.
The U.S. CDC has issued travel health notices for outbreaks in Mauritius, Mayotte, Réunion, Somalia, and Sri Lanka to warn international travelers of the Chikungunya health risk. Vaccination is recommended for travelers visiting an area with an outbreak.