Since no U.S. Food and Drug Administration (FDA) vaccines are available to prevent Alzheimer's Disease (AD), can other vaccines reduce the risk of this old-age disease?
The Conversation published an article written by neurodegenerative disease experts on January 18, 2023, highlighting the potential value of existing FDA-approved vaccines to reduce AD aggravating factors from viral infections.
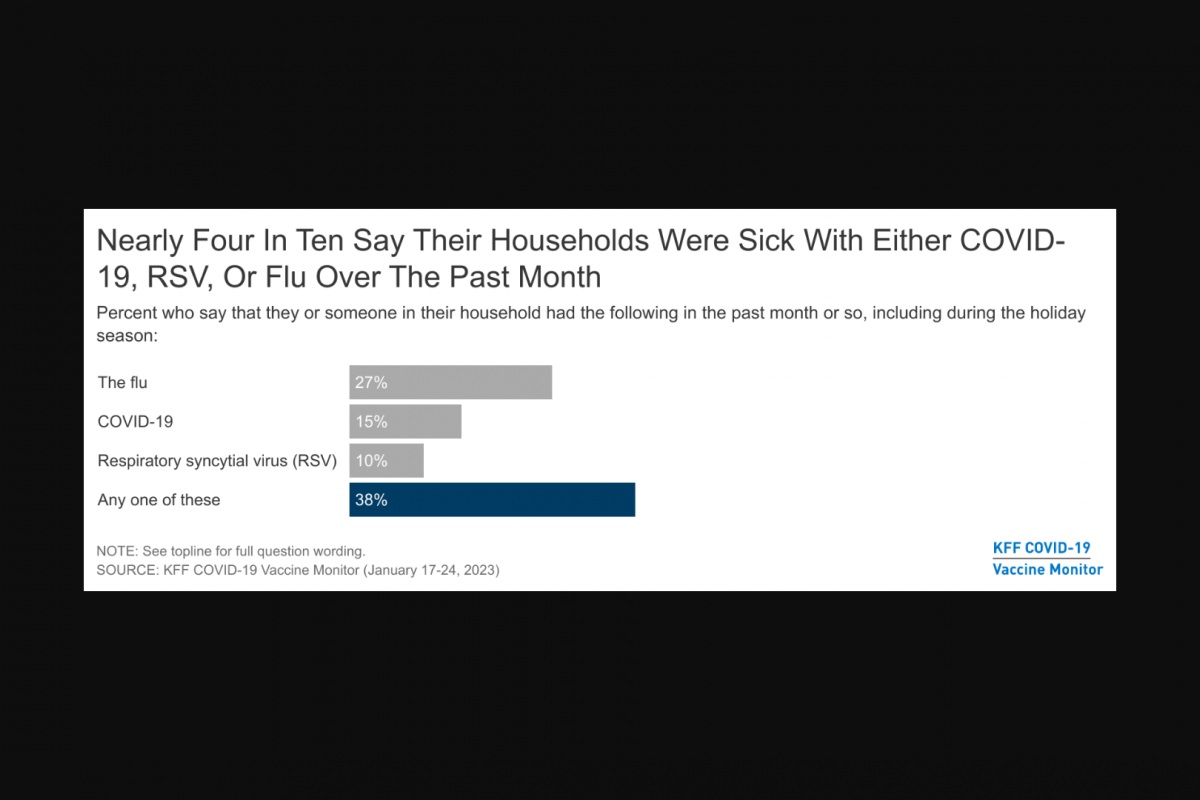
In previous studies, researchers identified that infections by varicella zoster (chickenpox and shingles) and influenza viruses could lead to a higher risk of AD.
However, researchers have been unable to consistently detect these suspect viruses in the brains of people who died of Alzheimer's.
In other words, when researchers analyze AD patient brains, detectable viral components may have disappeared, and causation is difficult to establish.
The full unedited article published by The Conversation is posted at this link.
As of February 12, 2023, the FDA has not authorized any of the current Alzheimer's vaccine candidates.