Advanced Head and Neck Cancer Clinical Study Expands
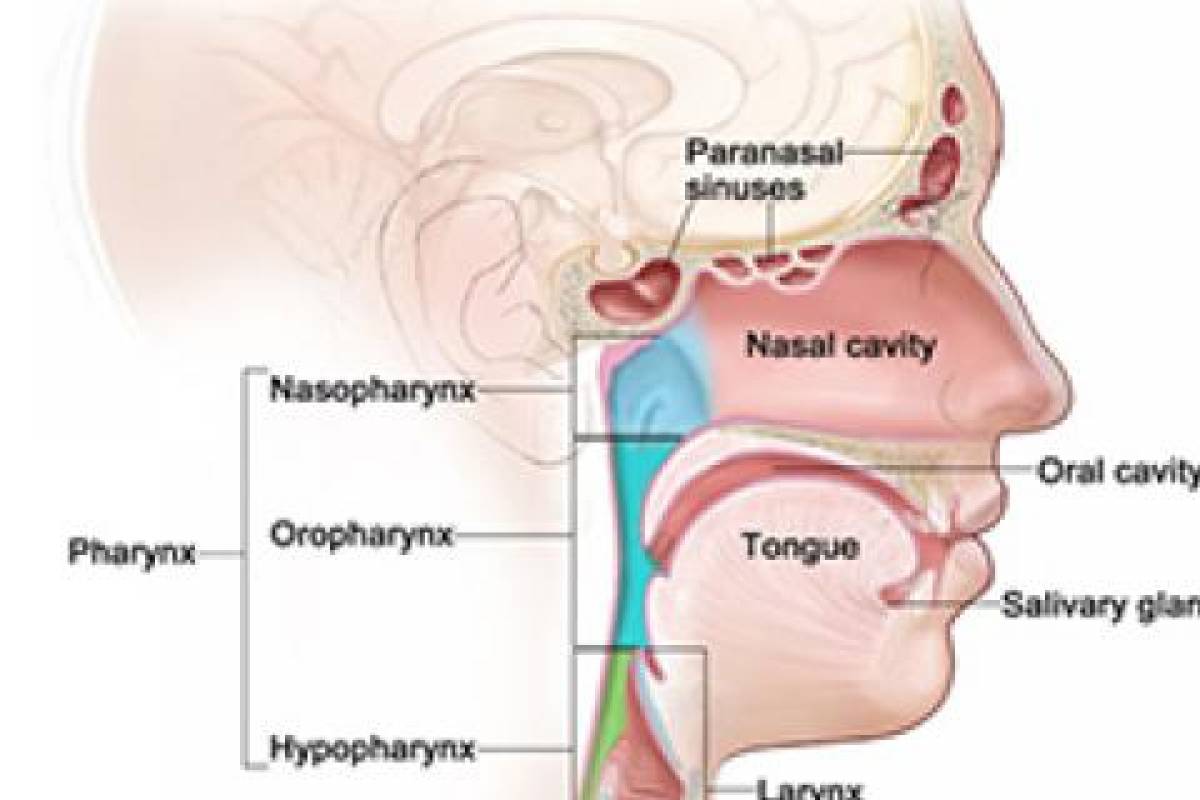
A biotechnology company developing immunotherapies and vaccines against cancers and infectious diseases announced today that its clinical trial of Gedeptin® for patients with recurrent head and neck cancers is now actively enrolling patients at three major research centers in the U.S.
GeoVax Labs, Inc.'s phase 1/2 trial (NCT03754933) is evaluating the safety and efficacy of repeat cycles of Gedeptin therapy in patients with recurrent head and neck squamous cell carcinoma (HNSCC), with tumor(s) accessible for injection and no curable treatment options.
A recent phase 1 dose-ranging study evaluating the safety of a single cycle of Gedeptin therapy found the therapy well-tolerated, with evidence of a reduction in tumor size in patients with solid tumors.
David Dodd, GeoVax President and CEO, commented in a press release on February 7, 2023, "The support of the U.S. FDA and collaborations with Stanford, Emory, and Jefferson enable us to evaluate Gedeptin rapidly in 10 patients, with the potential to expand the trial to 25 patients subsequently."
"A successful outcome may lead to labeling discussions with the FDA and initiation of further Gedeptin investigations, including in combination with immune checkpoint inhibitors, for additional cancerous and non-cancerous tumor indications."
Most patients are diagnosed with locally advanced disease and treated with surgery, chemotherapy, and radiotherapy. About 50% of these patients will experience a recurrence of the disease.
The GeoVax study is partially funded by the FDA under its Orphan Products Clinical Trials Grants Program.
The FDA has also granted Gedeptin orphan drug status for the intra-tumoral treatment of anatomically accessible oral and pharyngeal cancers, including cancers of the lip, tongue, gum, floor of the mouth, salivary gland, and other oral cavities.
Our Trust Standards: Medical Advisory Committee







