Malaria Prevention with Monoclonal Antibody Treatment
According to research published in The Lancet Infectious Diseases by researchers at the University of Maryland School of Medicine (UMSOM), a monoclonal antibody (mAbs) treatment conferred protection in people against P falciparum (malaria) at low doses
Published on January 25, 2023, this phase 1 clinical study found the mAbs CIS43LS protected 18 (82%) of 22 participants who received a dose. In addition, no participants developed parasitemia following dosing at 5 mg/kg intravenously or subcutaneously or at 10 mg/kg intravenously or subcutaneously.
All six control and four of seven participants dosed at 1 mg/kg intravenously developed parasitemia after controlled human malaria infection.
"The study demonstrates the feasibility of using mAbs therapies to help prevent malarial infection and holds promise for deployment to places where the disease is endemic," said Kirsten Lyke, MD, at UMSOM, in a related press release.
"This may allow us to revisit malaria eradication efforts."
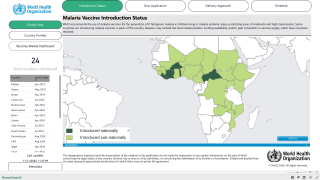
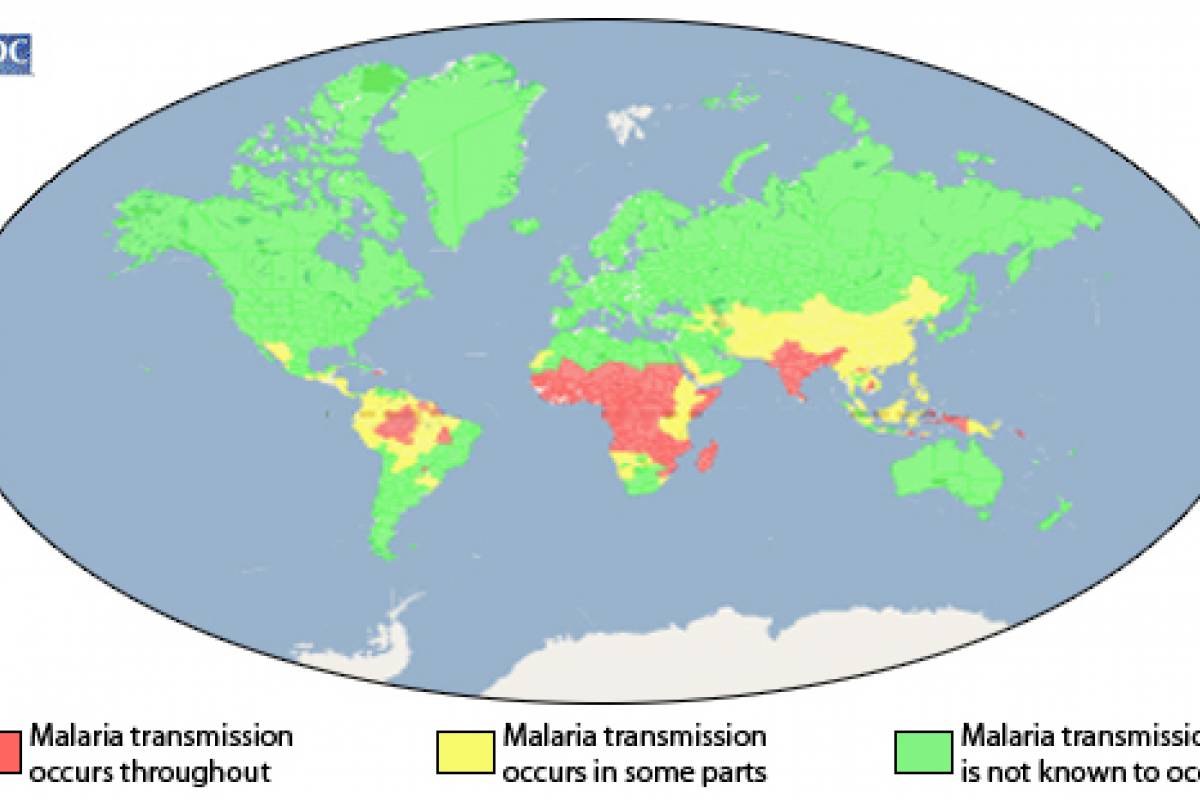
According to the World Health Organization (WHO), malaria is a vaccine-preventable disease caused by a parasite.
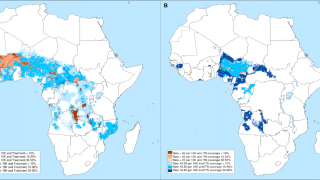
Vaccines like Mosquirix™ (RTS,S), and R21/Matrix-M™ have been reported to be effective at preventing disease in Africa and India.
As of February 11, 2023, the U.S. Food and Drug Administration (FDA) had not approved a malaria vaccine.
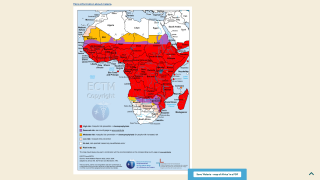
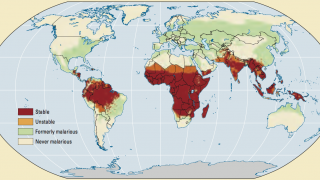
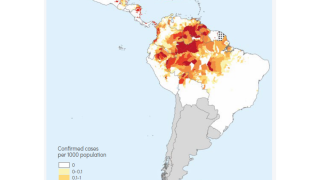
According to the 2021 World Malaria Report, about half the world's population lives in areas at risk of malaria transmission.
There were 241 million malaria cases and 627,000 deaths reported worldwide in 2020 alone, a 12% increase from 2019.
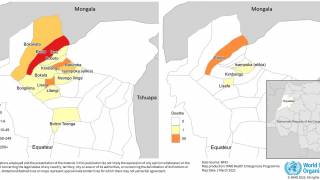
However, outbreaks of locally transmitted malaria cases in the U.S. have been limited and relatively isolated. The FDA has approved Artesunate to treat severe malaria in adult and pediatric patients.
Malaria outbreak news is posted at Vax-Before-Travel.com/MalariaOutbreasks.
Our Trust Standards: Medical Advisory Committee