The Kingdom of Lesotho recently confirmed a measles outbreak in the capital city of Maseru, which has a population of over 300,000.
In response, through the Maseru District Health Management Team, the Ministry of Health launched a measles vaccination campaign in mid-April 2023.
Measles is a severe disease that can be prevented with safe and effective vaccines.
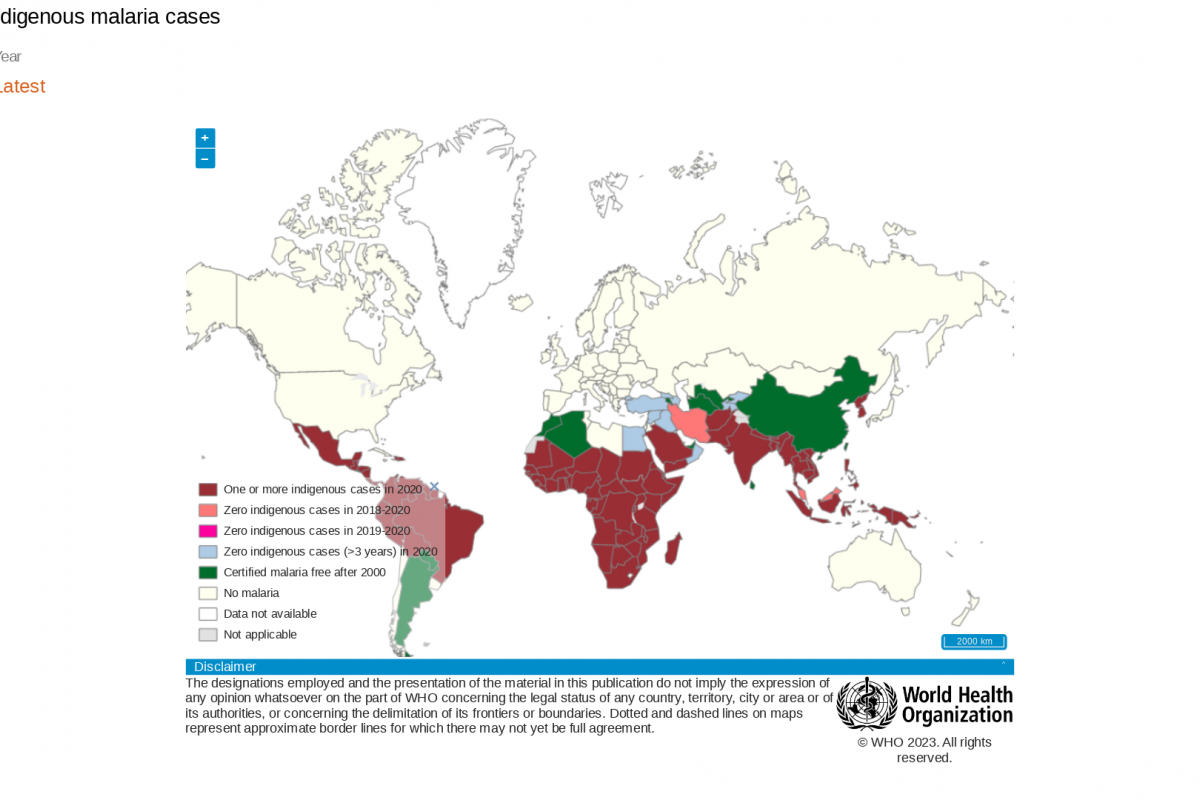
Lesotho is located in the country of South Africa, which has been reporting measles outbreaks in 2023.
South Africa's National Institute for Communicable Diseases reported in April the percentage of samples testing positive for measles remained at 27%.
Unfortunately, the Mountain Kingdom's 2022 measles vaccination campaign turnout was about 33% in 2022.
Before 2019, Lesotho had gone about ten years without a measles outbreak.
Measles outbreak news and alerts are posted by Vax-Before-Travel.