Mpox Vaccination Adverse Events Explained

A recent Research Letter published by the JAMA Network found that local adverse event rates were highest following intradermal administration of the Mpox vaccine.
During the early stages of the Mpox outbreak in 2022, many countries adopted a dose-sparing schedule with 0.1-mL intradermal MVA-BN (JYNNEOS®) vaccine recommended for the preexposure and 0.5-mL subcutaneous vaccine for postexposure prophylaxis, two doses given four weeks apart.
As reported on May 4, 2023, the adverse event rate was highest following dose one of intradermal vaccination (53%) and lowest following dose two of subcutaneous vaccination (31%).
The most common adverse events were local redness, itching, and swelling following intradermal vaccination and local pain, swelling, and redness following subcutaneous vaccination.
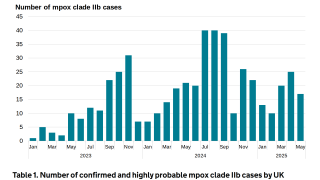
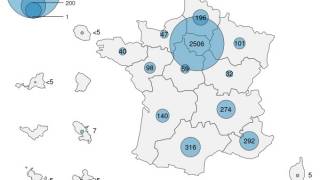
During May 2023, the JYNNEOS vaccine is readily available in most countries. Furthermore, there has been a resurgence of Mpox cases in various cities, such as Chicago, Paris, and Seoul.
Our Trust Standards: Medical Advisory Committee