The World Health Organization (WHO) recently reported, based on the current data and available information from the Indonesia Ministry of Health, the overall risk of measles at the national level is assessed as high.
And the WHO reconfirmed measles is endemic in Indonesia and is an ongoing risk around the world.
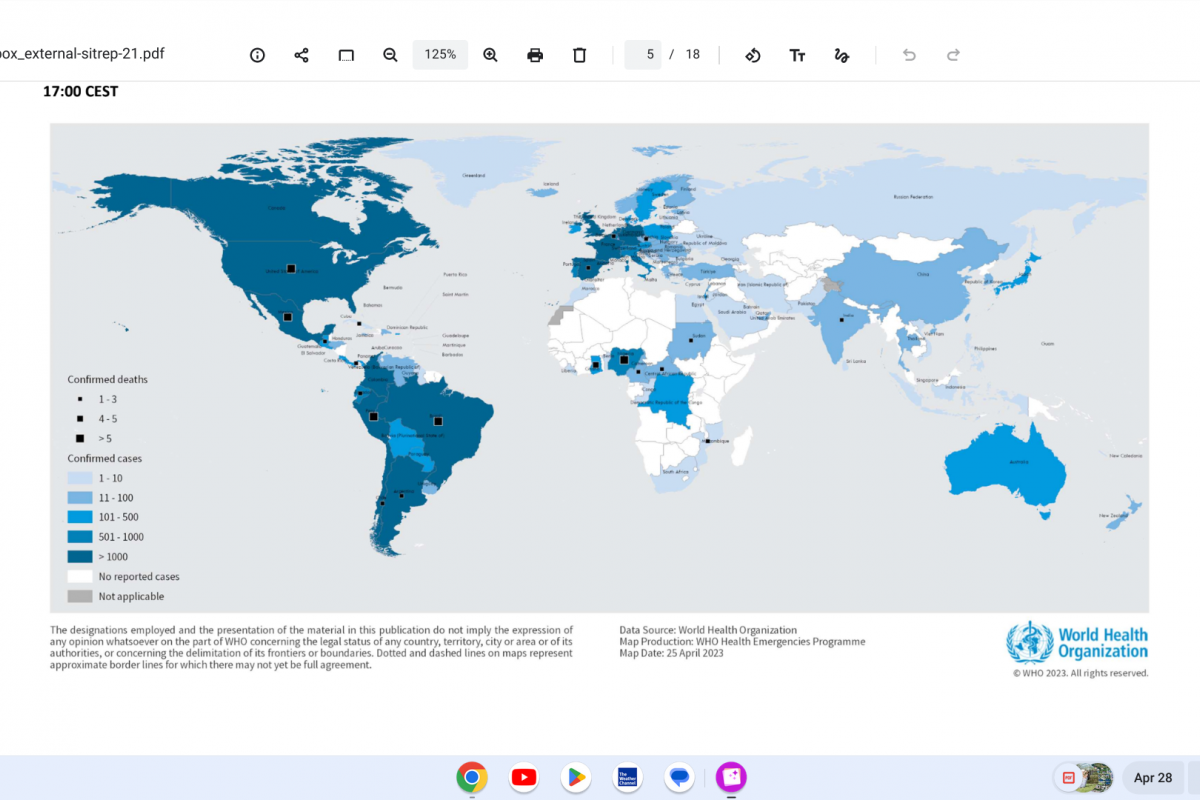
On April 28, 2023, the WHO reported a total of 2,161 measles cases had been reported across 18 of 38 provinces in Indonesia, primarily from the provinces of West Java (796 cases) and Central Papua (770 cases) from January thru April 3, 2023.
In 2022, a total of 4,845 laboratory-confirmed measles cases and six deaths were reported across 32 of the 38 provinces.
To alert international visitors, the U.S. CDC issued a Watch - Level 1, Practice Usual Precautions regarding the global measles outbreak, which included Indonesia.
Recently, American Samoa's government declared a measles outbreak.
Measles is caused by a highly contagious virus that spreads through the air by direct contact with infectious droplets or by airborne spread when an infected person breathes, coughs, or sneezes. The measles virus can live for up to two hours in airspace after an infected person leaves an area.
Furthermore, the CDC says that measles can be severe in all age groups and can lead to serious complications, such as pneumonia.
And all international travelers, including infants and children, should be fully vaccinated against measles.
Measles vaccines are generally available at clinics and community pharmacies in the U.S.