Vakzine Projekt Management GmbH (VPM) today announced the successful licensing of the novel R21/Matrix-MTM Malaria Vaccine by the Ghana Food and Drugs Authority.
On May 10, 2023, VPM confirmed in a press release that the R21/Matrix-M™ vaccine has emerged as the most effective vaccine against malaria.
This innovative malaria vaccine was initially developed by the lab research team of Adrian Hill, Director of the University of Oxford's Jenner Institute.
Based on available clinical data in 2023, the R21/Matrix-M™ vaccine's efficacy is greater than 75%, far above the effectiveness of the other approved malaria vaccine, Mosquirix™ (RTS,S/AS01).
Notably, the manufacturing capacity of 200 million doses/year by SII will increase the current supply capacities by >20-fold globally.
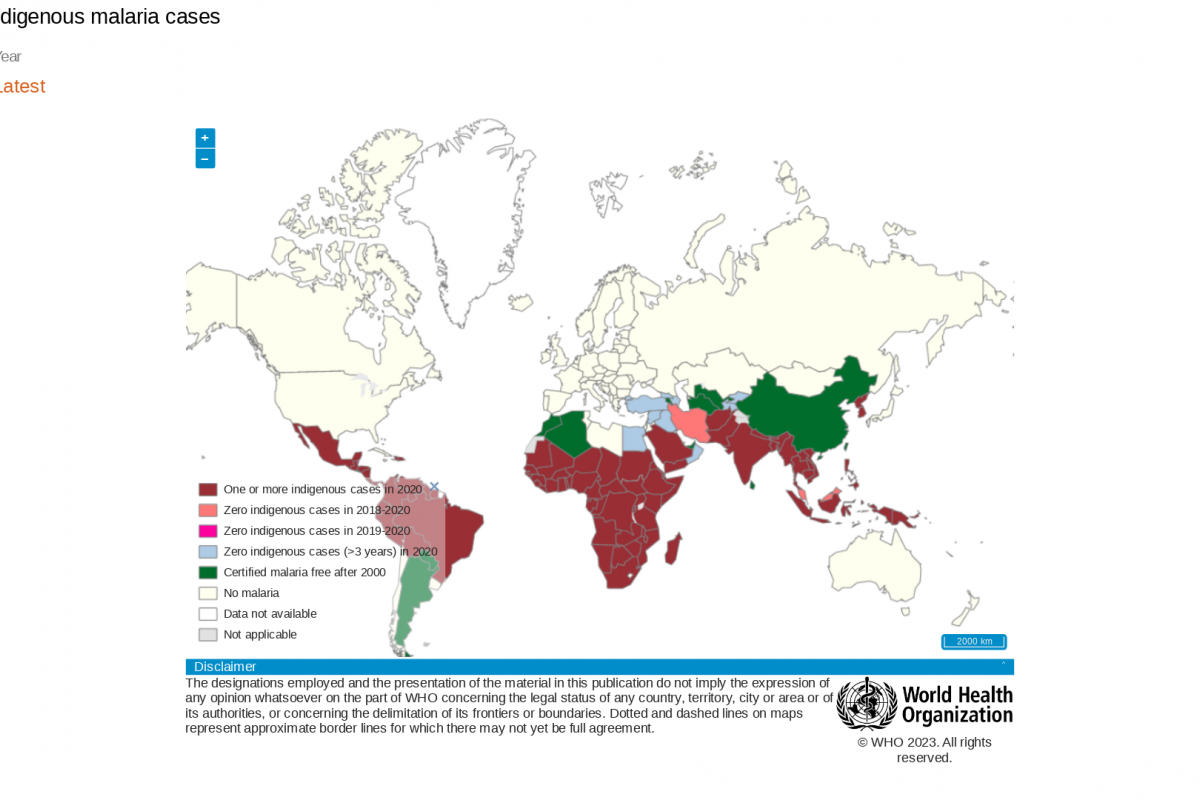
Malaria is one of the leading causes of pediatric morbidity and mortality in sub-Saharan Africa. And that children under five account for approximately 80% of all malaria-related fatalities, says the WHO Africa.
Four African countries accounted for just over half of all malaria deaths worldwide: Nigeria (31.3%), the Democratic Republic of the Congo (12.6%), the United Republic of Tanzania (4.1%), and Niger (3.9%).
And in the Northern Hemisphere, Costa Rica recently reported 105 positive malaria cases.
Malaria outbreak news is posted by Vax-Before-Travel.