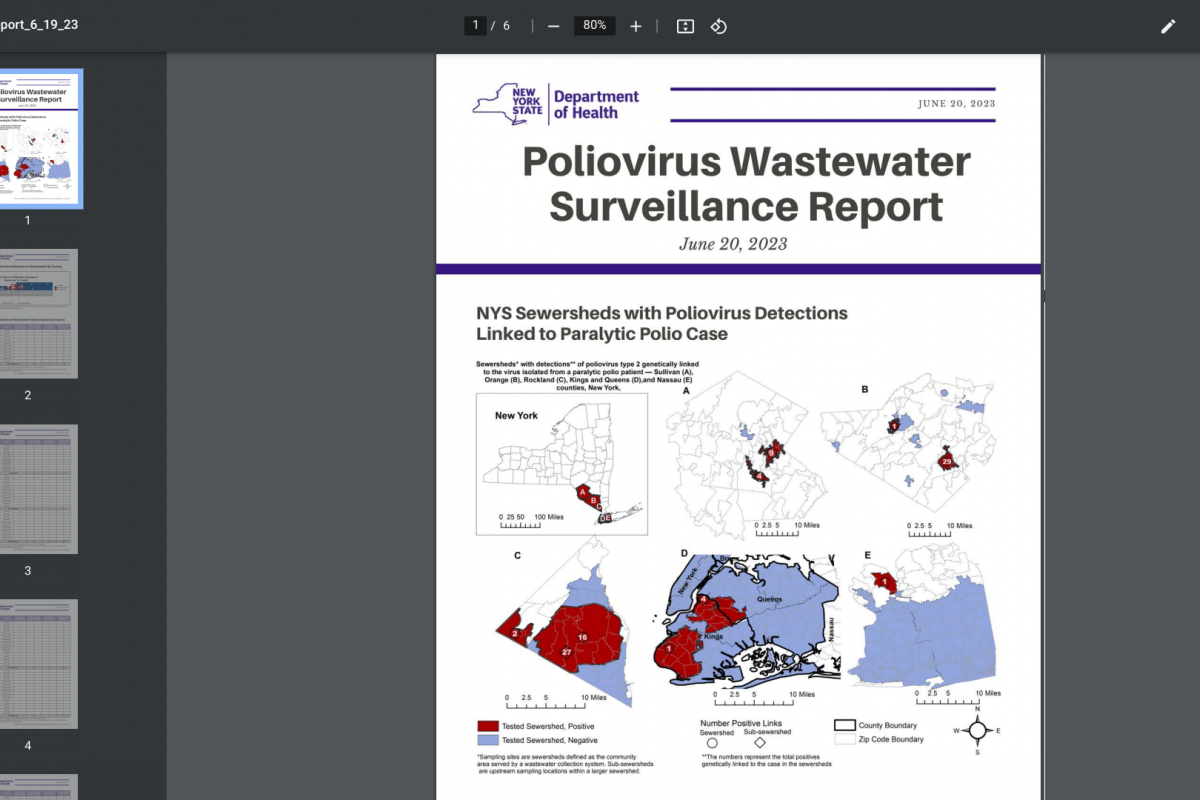
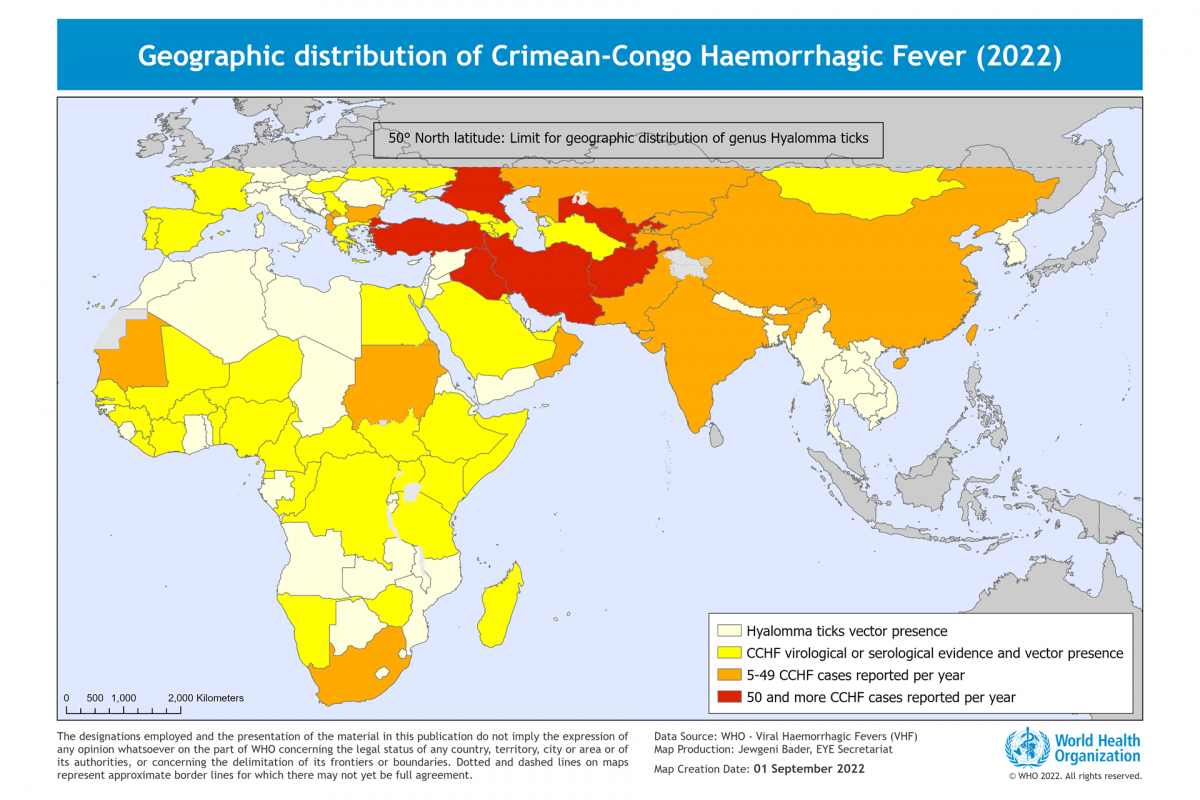
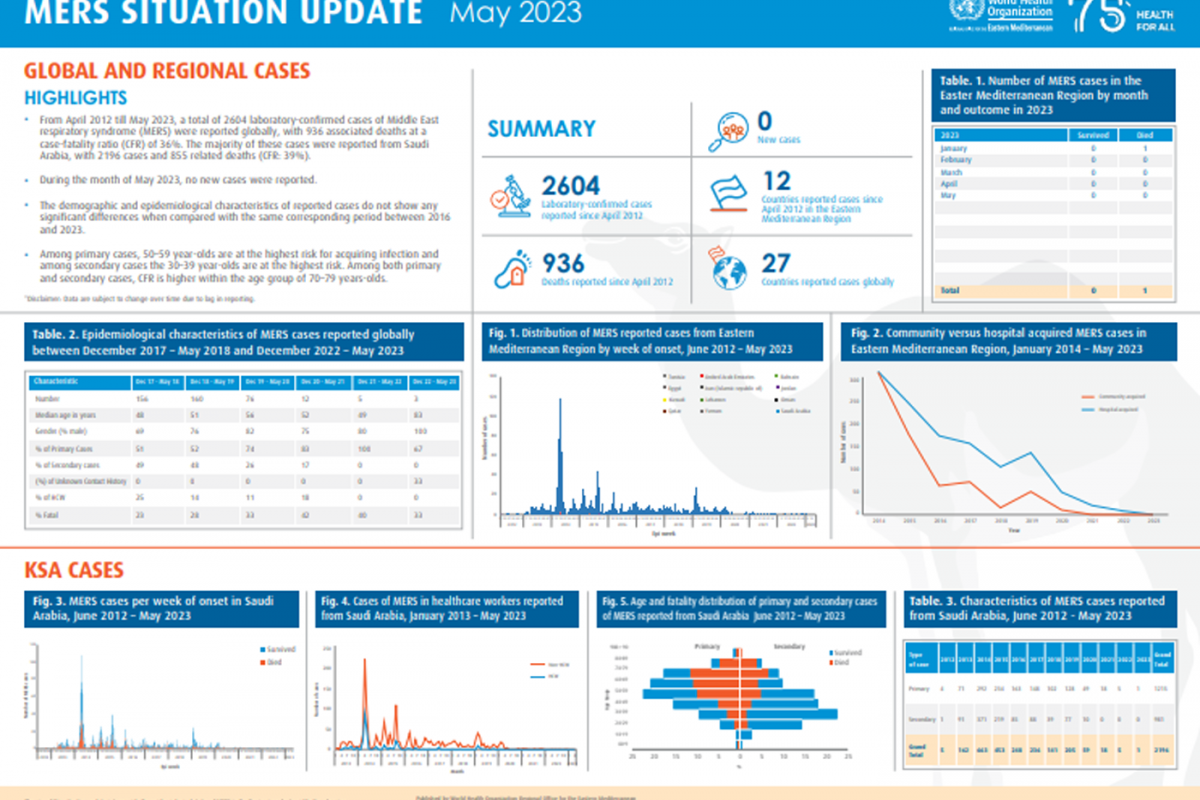
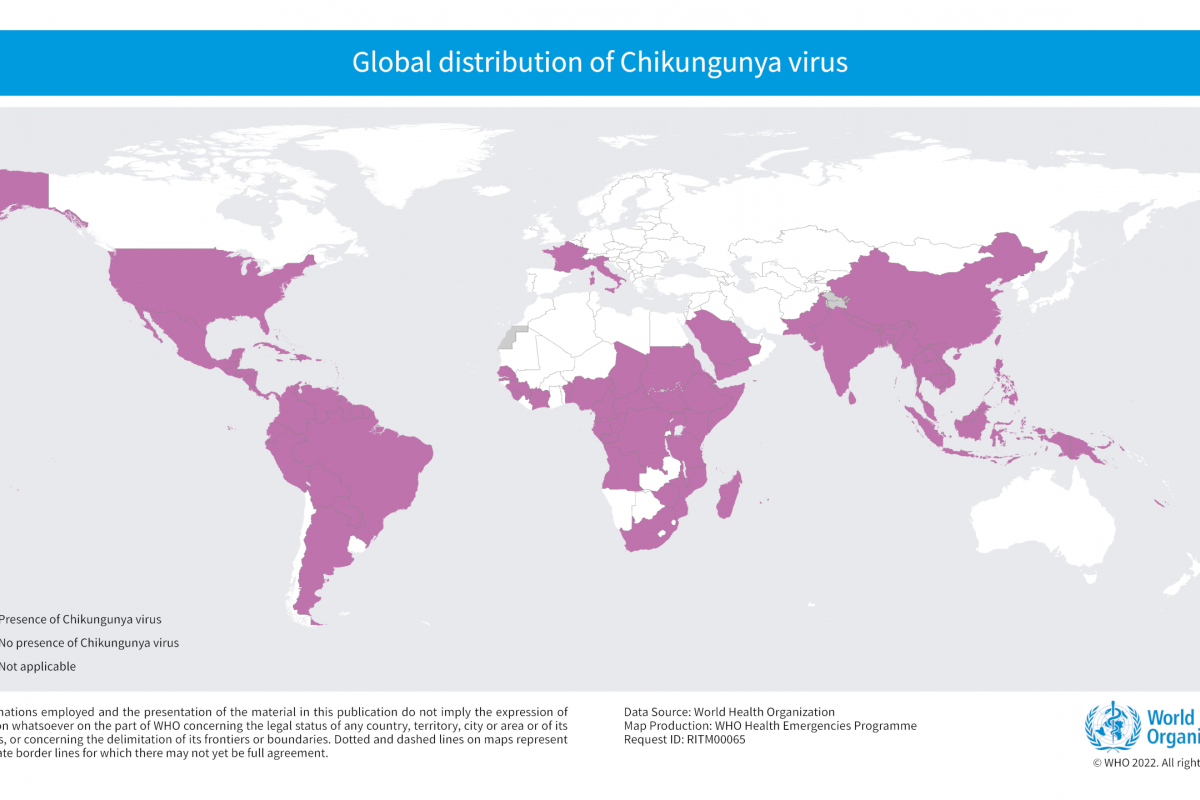
Invivyd, Inc. recently announced positive initial data from its ongoing Phase 1 clinical trial of its lead investigational monoclonal antibody (mAb) candidate, VYD222.
VYD222 is a broadly neutralizing, half-life extended mAb candidate in development for the prevention of symptomatic COVID-19 in vulnerable populations, such as immunocompromised people.
Initial Phase 1 data show that a single administration of VYD222 was generally well-tolerated at all three dose levels tested, with no serious adverse events reported to date.
At the lowest VYD222 dose tested (1500 mg), geometric mean serum neutralizing titers were 3245.1 (95% CI: 1882.5, 5594.0) against Omicron XBB.1.5 at Day 7, with a geometric mean 38.87-fold rise (95% CI: 10.3, 146.8) from baseline to Day 7 (n=8).
Greater VYD222 dose levels are designed to provide greater protection from any potential loss of neutralization activity as SARS-CoV-2 evolves.
Dave Hering, chief executive officer of Invivyd, stated in a press release on June 22, 2023, "Based on previously published clinical data from randomized controlled clinical trials, we believe that mAb directed against the receptor binding domain of the SARS-CoV-2 spike protein offer an attractive safety profile, even at higher doses, and that strong serum neutralization activity would be predictive of clinical benefit."
Additional COVID-19 monoclonal antibody news is posted by Precision Vaccinations.