Poland's Chief Veterinary Office recently confirmed 29 samples of avian influenza A(H5N1) were tested, 20 of which came from cats from Gdańsk, Gdynia, Poznań, Lublin, Pruszcz Gdański, Nowy Dwór Mazowiecki, Bydgoszcz, Wrocław, the Rzeszów district and the vicinity of Zamość.
As of June 30, 2023, Poland's State Veterinary Institute in Puławy reported these infections. Previously, a team of scientists from the Faculty of Veterinary Medicine of the Warsaw University of Life Sciences examined one of the samples.
Since June 23, 2023, media sources have referred to at least 70 domestic cat deaths in Poland, for which investigations are ongoing, reported the European Centre for Disease Prevention and Control (ECDC).
'Several uncertainties currently exist regarding the source of infection, the potential of feline-to-feline and feline-human transmission of the particular A(H5N1) influenza virus strain, and the severity of the disease.
Furthermore, no human cases have been reported related to this (cat) event in Poland, wrote the ECDC.
The U.S. CDC published an updated report on June 30, 2023, to include information on additional sporadic human cases and activity in wild birds, poultry, and other animals.
The overall risk to human health associated with the ongoing outbreaks of highly pathogenic A(H5N1) viruses in wild birds and poultry has not changed and remains low at this time, says the CDC.
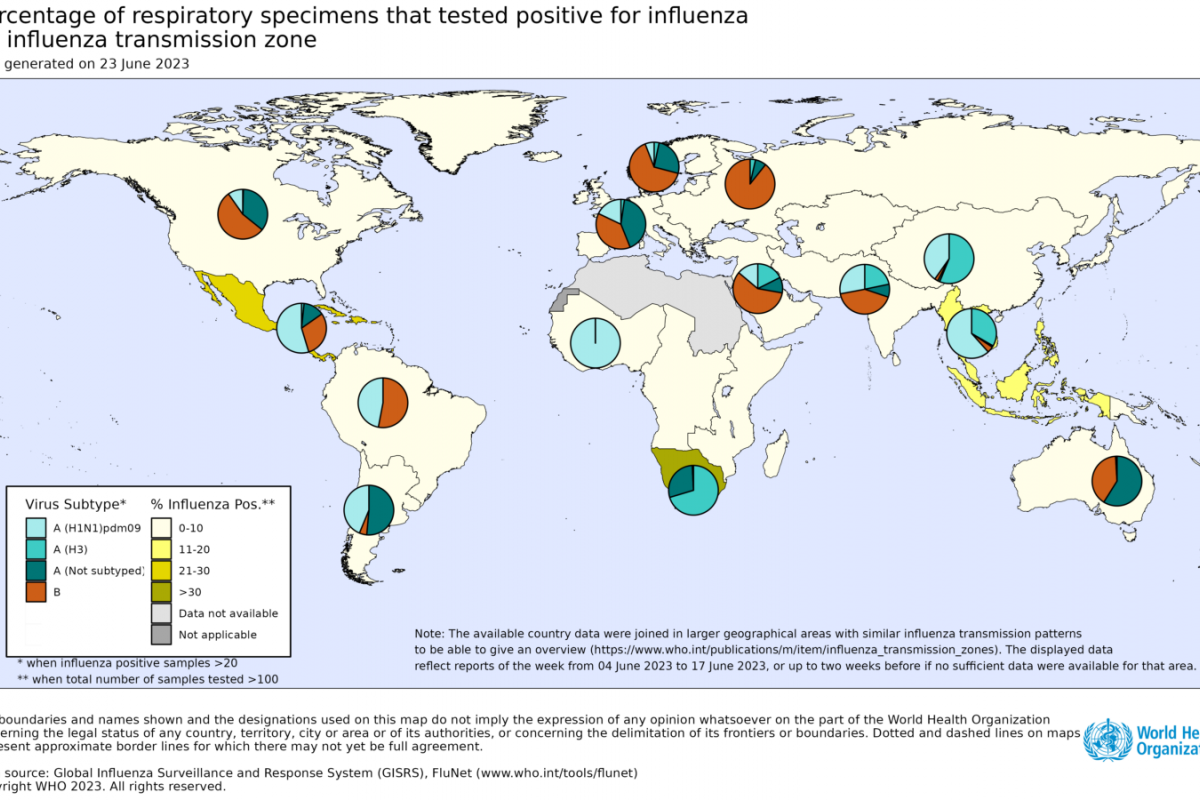
Bird flu outbreaks have been confirmed worldwide.
According to the CDC FluView dashboard, 54 countries reported bird flu outbreaks to the World Health Organization from 2021 through June 2023.
As of July 1, 2023, the U.S. government continues to fund bird flu vaccines.