The U.S. Centers for Disease Control and Prevention (CDC) today republished an expanded global polio outbreak Travel Health Notice.
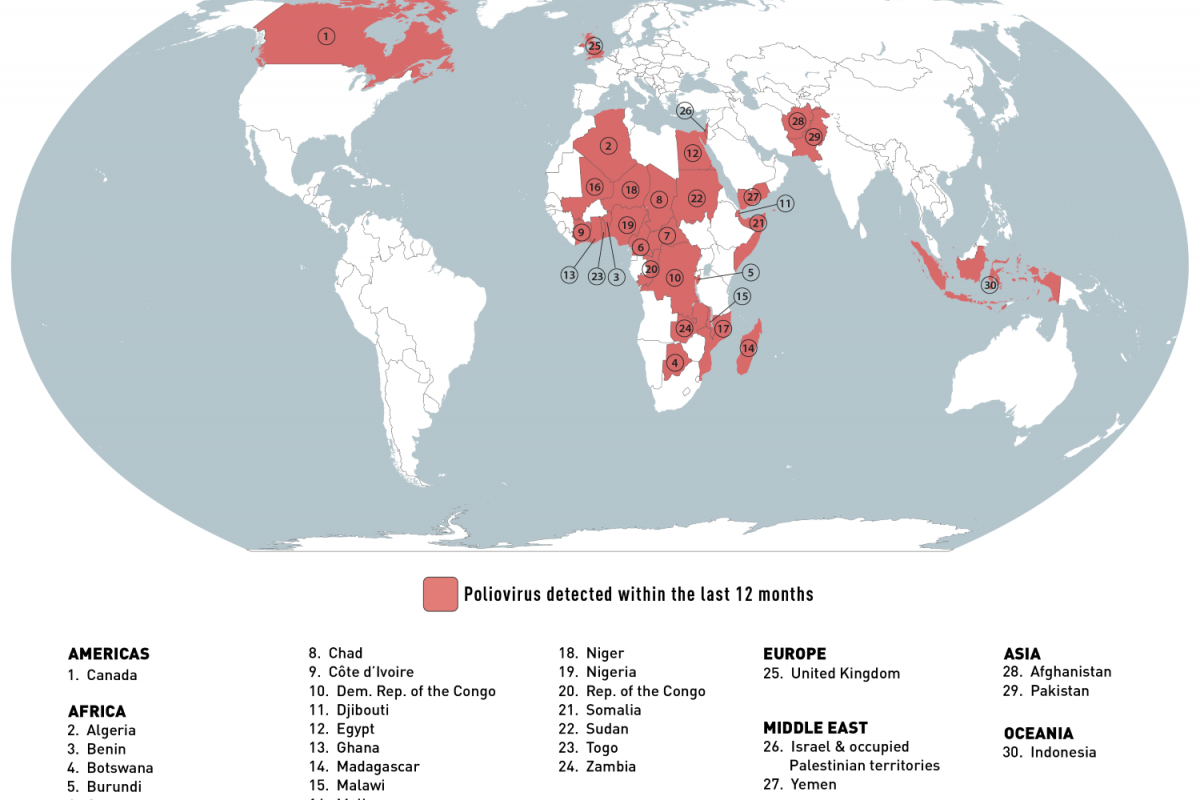
On June 26, 2023, the CDC identified thirty destinations with circulating poliovirus.
And, before travel to any destination listed, adults who previously completed the full, routine polio vaccine series may receive a single, lifetime booster dose of a polio vaccine.
In the U.S., the IPV vaccine has been offered since 2000. Oral polio vaccines are provided in various countries in 2023.
For example, the new nOPV2 vaccine has been administered over 620 million times in recent years.
The CDC says polio is a crippling and potentially deadly disease that affects the nervous system.
Because the virus that causes polio lives in the feces of an infected person, people infected with the disease can spread it to others when they do not wash their hands well after defecating.
People can also be infected if they drink water or eat food contaminated with infected feces.
Most people with polio do not feel sick. Some people have only minor symptoms, such as fever, tiredness, nausea, headache, nasal congestion, sore throat, cough, stiffness in the neck and back, and pain in the arms and legs.
In rare cases, polio infection causes permanent loss of muscle function. Polio can be fatal if the muscles used for breathing are paralyzed or if there is an infection of the brain, says the CDC.