Dengue Outbreaks
Dengue Outbreaks September 2025
According to the World Health Organization (WHO) and numerous health agencies, Dengue is a viral infection spread to humans by infected mosquitoes of the Aedes genus and a leading cause of febrile illness among international travelers in 2025. The WHO has classified Dengue as a grade 3 emergency, with an estimated 4 billion people at risk globally. The WHO states that Dengue is a vaccine-preventable disease, endemic in approximately 110 countries, including areas within the United States, such as Florida and Puerto Rico.
As of August 21, 2025, the WHO Dengue Situation Update 728 indicates that over 6 million Dengue cases and 7,552 related fatalities have occurred in 2025. More than 13 million dengue cases were reported in 2024, marking the highest number of cases on record.
On January 20, 2025, the WHO published a Global Strategic Preparedness, Readiness, and Response Plan for Dengue. Dengue's four subviruses are usually spread to people through the bites of infected Aedes mosquitoes. On October 3, 2024, the WHO launched the Global Strategic Preparedness, Readiness, and Response Plan to tackle Dengue and other Aedes-borne arboviruses. The WHO plan aligns with the Global Vector Control Response 2017-2030, a global strategy to strengthen vector control worldwide, and the Global Arbovirus Initiative.
An analysis published in July 2024 reported that the most frequent regions of dengue infection acquisition were Southeast Asia (50.4%), South Central Asia (14.9%), the Caribbean (10.9%), and South America (9.2%). The median age was 33 years, and tourism was the most frequent reason for travel (67.3%). An analysis published in December 2024 revealed a clear link between climate change and the expansion of vectors, such as mosquitoes, into new territories, increasing disease incidence. Mosquitoes that spread Dengue viruses usually live below 6,500 feet; therefore, a person's chances of getting Dengue in high altitudes are very low.
Dengue Outbreak Travel Advisories
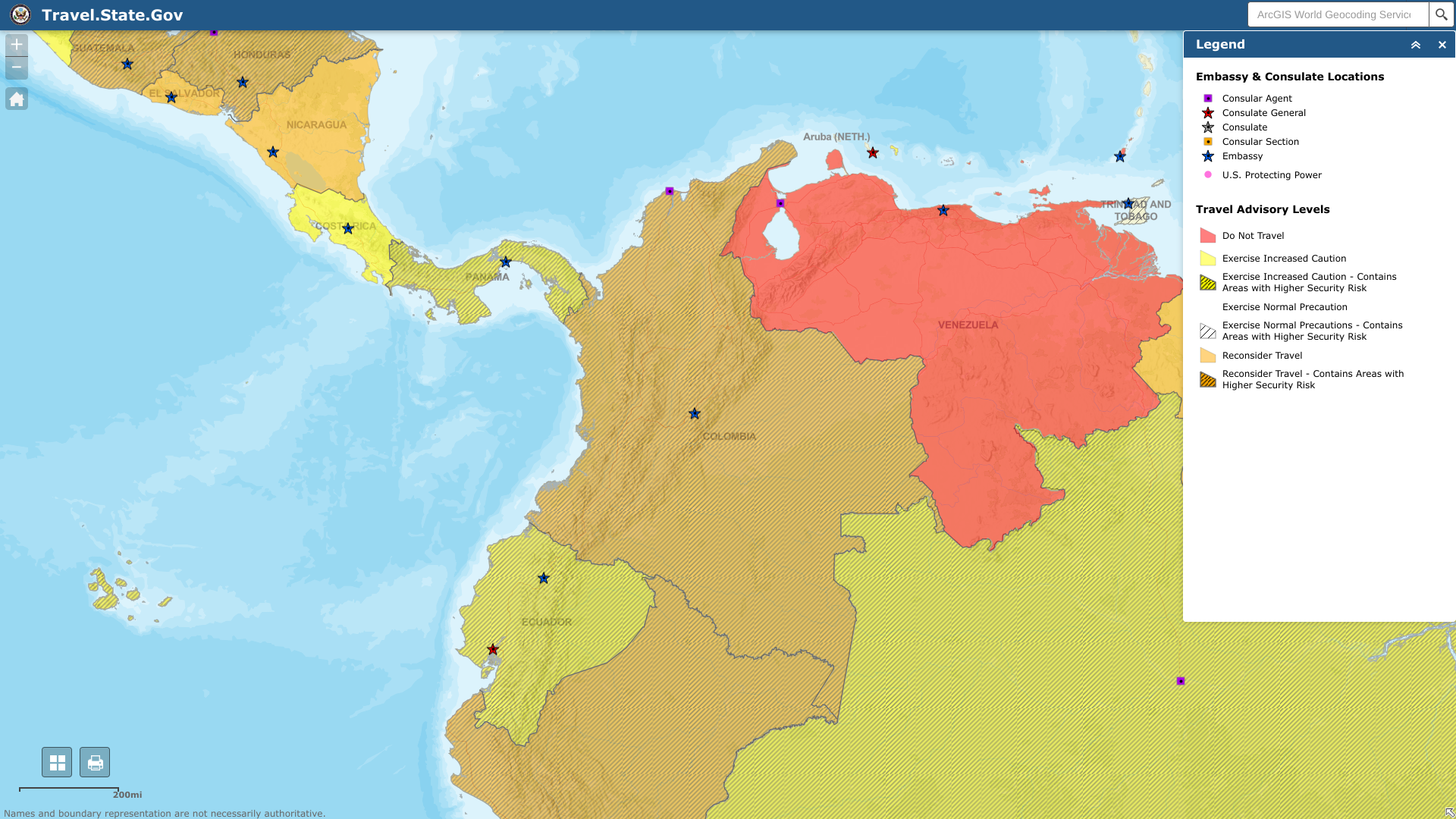
The U.S. Centers for Disease Control and Prevention (CDC) reissued a Global Travel Health Notice on August 21, 2025, regarding Dengue outbreaks in the Americas, Africa/Middle East, and Asia/Pacific regions, such as India, Singapore, Thailand, the Philippines, Malaysia, and Myanmar. The CDC has not issued travel advisories for U.S. states that have reported local dengue outbreaks, such as Florida and Puerto Rico. As of August 2025, HealthMap has published dengue case maps.
Dengue Outbreak in the United States
As of July 2025, the U.S. CDC advises clinicians to consider Dengue in patients with fever who live in or have recently traveled to areas with a risk of Dengue. The CDC reported in September 2025 that 3,045 Dengue cases occurred in 47 jurisdictions this year. The CDC says transmission of Dengue virus serotypes (DENV-1, 2, 3, 4) remains high in the U.S. territories of Puerto Rico and the U.S. Virgin Islands. DENV-3 is the most common (84%) serotype identified in 2025.
In 2024, 53 jurisdictions, led by Arizona, California, Florida, New Jersey, New York, and Puerto Rico, reported 9,391 dengue cases. In June 2024, the CDC issued an updated Health Alert Network Health Advisory, notifying healthcare providers, public health authorities, and the general public of an increased risk of dengue virus infections in the United States. In 2023, 52 U.S. jurisdictions reported 6,164 dengue cases to the CDC.
As of December 2024, the Florida Department of Health (FDH) reported over 999 travel-associated and 91 locally acquired dengue cases throughout the state, with the majority occurring in Miami-Dade County.
The Texas Department of State Health Services (DSHS) reports that mosquitoes that transmit dengue fever are present in the state of Texas. As of September 2025, DSHS reported 31 travel-related dengue cases this year. As of December 2024, there were 43 imported dengue cases in 23 Texas counties, led by Travis County (18), and one local case in Cameron County, with one related fatality. Texas reported 79 travel-related dengue cases in 2023 and one locally acquired case in Val Verde County.
In California, the San Bernardino County Public Health Department reported (1) a locally acquired case of Dengue in San Bernardino on November 7, 2024. The Los Angeles County Department of Public Health has reported 12 locally acquired dengue cases in the San Gabriel Valley, specifically in the cities of Baldwin Park (8), El Monte (2), Hollywood Hills (1), and Panorama City (1), in 2024. Dengue cases were reported in San Diego, Escondido, and Vista in 2024. Over 360 dengue cases were confirmed in California in 2023. California reported two locally acquired cases (in Long Beach and Pasadena) and 250 travel-related cases.
Between 2010 and 2023, 250 locally acquired cases were reported in Hawaiʻi.
Dengue Outbreak U.S. Territories
The CDC says the Dengue virus is endemic in the U.S. territories of Puerto Rico, American Samoa, the U.S. Virgin Islands, the Federated States of Micronesia, the Republic of the Marshall Islands, and the Republic of Palau. On July 7, 2025, American Samoa declared a public health emergency due to a surge in dengue cases. In the U.S. Virgin Islands, a dengue outbreak was declared in August 2024 and remains in effect in 2025. A total of 208 locally acquired cases were identified in 2024 and 30 in 2025, all on the island of St. Croix. The Virgin Islands Department of Health urged residents to take immediate precautions to prevent further dengue transmission as cases surged in the St. Thomas-St. John's area. John's area. John's area. John's area. John's area. John's area. John's area. John's area. John's area. John's area. John and St. Croix Districts.
In March 2024, the Health Ministry declared a public health emergency in Puerto Rico, which remains in effect as of 2025. As of March 7, 2025, 936 cases have been reported, representing a 113% increase compared to the same period in 2024. In 2024, 6,291 cases were reported, and 13 deaths were reported. Puerto Rico's Department of Health confirmed that Dengue was endemic in the greater San Juan area, with 4,467 cases.
Among American Samoa school-aged children, the estimated seroprevalence was 59% for those aged 7–16.
Dengue Outbreaks in Africa
Dengue circulation has been detected in more than 30 African countries. According to the Africa CDC Epidemic Intelligence Report, as of January 2025, over 1,300 dengue cases have been reported in Africa. As of 2024, over 74,000 dengue cases have been reported this year from Burkina Faso, Cameroon, Cabo Verde, Central African Republic, Chad, Côte d'Ivoire, Ethiopia, Ghana, Kenya, Mali, Mauritius, São Tomé and Príncipe, Senegal, Sudan, and Togo. The ACDC reported in 2024 that travelers visiting these African countries may be at an increased risk of Dengue. In 2023, 171,991 dengue cases and 753 deaths were reported in African countries. The U.S. CDC issued a Travel Health Notice in 2023, confirming Dengue is an ongoing risk in Africa. A December 2025 study found that countries in eastern Africa had a high estimated risk of dengue importation from Asia and other countries in east Africa. In contrast, for West African countries, the risk of importation was higher from within the region than from countries outside of Africa.
Dengue Outbreaks in the Americas
The first suspected dengue-like epidemics were reported in 1635 in Martinique and Guadeloupe. The Pan American Health Organization (PAHO) issued an Epidemiological Alert in February 2025, based on the growing circulation of DENV-3, which had not been circulating previously, thereby increasing the probability of severe cases. In epidemiological week 34 of 2025, a total of 3,740,133 suspected dengue cases were reported. This data represents a 68% decrease compared to the same period in 2024. As of December 31, 2024, 49 countries and territories in the Americas reported over 13,017,982 Dengue cases and 8,151 related deaths in 2024. In 2023, 4,617,108 Dengue cases were reported in the Americas.
Argentina's Epidemiological Bulletin reported over 580,000 dengue cases in 2024, with 56,435 in Buenos Aires. Argentina's Ministry of Health published a Comprehensive Preparedness and Response Plan for Dengue Epidemics 2024-2025.
Over the past 25 years, nearly 18 million Brazilians have contracted one of the four viruses that cause Dengue. Brazil's Ministry of Health reports indicate that, as of 2024, there were over 9.6 million dengue cases and 5,441 related deaths. The São Paulo state dengue data dashboard was updated in May 2025. A study published in The Lancet in March 2025 forecasts that Brazil's Dengue outbreaks will refocus on the southern mountain area. The Brazilian Ministry of Health reported dengue cases among pregnant women reached 5,151 in the first six weeks of 2024, compared to 1,157 in the same period in 2023. In 2023, Brazil reported approximately 2.9 million patients, a 20% increase from the previous year, and Rio de Janeiro reported 22,959 dengue cases.
Dengue is hyperendemic in Colombia. It imposes a substantial economic burden on patients, caregivers, society, and the national health system. In 2025, Colombia's Huila Department declared a public health emergency.
According to the Health Surveillance Directorate of the Republic of Costa Rica, 1,076 dengue cases were reported in 2024. Costa Rica confirmed over 24,000 dengue cases in 2023, with the Huetar Caribe and Central Sur regions reporting the highest number of cases. As of August 4, 2023, all four dengue serotypes were registered.
In French Guyana, over 8,000 confirmed dengue cases have been reported since the beginning of 2024.
The U.S. CDC includes Mexico in its global Level 1 Dengue Travel Health Advisory. As of February 2025, Mexico has reported over 10,000 dengue cases. In 2024, Mexico confirmed about 549,000 dengue cases. A May 2024 model predicted that the percentage of municipalities affected by Dengue would rise from 55 to 91% in Mexico. Dengue was reported in 28 of 32 Mexican states in 2023, and transmission has been documented in Yucatan since 1979. All four DENV serotypes have been found in Mexico for decades. Between 1995 and 2008, constant circulation of DENV-3 was recorded in Mexico. Furthermore, notable increases in circulation were observed in 2022 (25%), 2023 (59%), and 2024 (86%).
In Nicaragua, 92,022 dengue cases were reported in 2024. A study published on January 10, 2025, demonstrates differences in dengue severity by serotype and immune status, emphasizing the critical need for a dengue vaccine with balanced effectiveness against all four serotypes, as existing vaccines show variable efficacy by serotype and serostatus.
DENV was reintroduced in Panama in 1993 after a 35-year absence of autochthonous transmission. All four serotypes were detected in Panama. As of April 2025, Panama reported over 4,800 Dengue cases. An analysis published in 2024 suggests that Panamanian strains were related to viruses from different regions of the Americas, suggesting a continuous exchange of viruses.
Paraguay's Ministry of Health confirmed that the DENV-3 subtype returned in 2025 after a nine-year absence. A study published in May 2025 suggests a high seroprevalence of DENV in Paraguayan blood donors. The high DENV seroprevalence reflects the impact of past Dengue outbreaks.
Peru issued an Alert in November 2024, notifying public and private health facilities of the increased risk of dengue infections. On November 23, 2024, a dengue vaccination program was launched in 16 districts of Loreto, Piura, Tumbes, and Ucayali regions.
On August 16, 2024, the Republic of Trinidad and Tobago confirmed 825 cases of Dengue Fever and eight (8) laboratory-confirmed deaths. The Caribbean reported over 62,000 dengue cases in 2023, a significant increase from 20,349 cases in 2022. Jamaica's Ministry of Health declared a dengue outbreak on September 23, 2023.
Dengue Outbreaks Australia 2025
In Australia, cases of locally acquired Dengue have historically been reported from most mainland Australian states. Dengue notifications have been reported monthly from 2019 to 2025. In January 2025, there were 261 Dengue case notifications. Queensland Health declared a Dengue outbreak centered in the Townsville City Local Government Area (TLGA) on February 19, 2025. As of March 25, 2025, 11 cases (9 confirmed and two probable) had been reported.
Dengue Outbreaks in Asia and the Pacific Islands
In 2025, the WHO confirmed that the Western Pacific Region continues to face a high burden of mosquito-borne arboviral diseases, particularly Dengue. In 2025, the U.S. CDC reported that countries in the WHO Western Pacific Region reported higher-than-usual dengue cases. Travelers visiting the following countries may be at increased risk: Bangladesh, Cambodia, Hong Kong, Indonesia, Fiji, Malaysia, Myanmar, Nepal, the Philippines, Samoa, Singapore, Sri Lanka, Tonga, and Thailand. The WHO publishes Dengue Situation Updates for the Western Pacific Region in 2025.
In 2025, the Cook Islands Ministry of Health declared a dengue fever outbreak in Rarotonga, following confirmation of seven dengue cases.
Fiji Ministry of Health & Medical Services confirmed one Dengue-related fatality on April 7, 2025. Between January and April 15, 2025, approximately 5,100 dengue cases were reported nationwide, with the Western Division leading with 2,077 cases. In 2014, the Fijian Government launched a cleanup campaign to combat the outbreak of dengue fever. Between November 2023 and April 2025, 1,721 dengue cases were reported in French Polynesia.
The Centre for Health Protection (CHP) in Hong Kong reported seven imported cases of dengue fever as of March 20, 2025. In 2024, 161 cases were imported (75 from Mainland China), and five local cases were reported. In 2023, 62 cases of DF were imported from abroad.
In September 2024, the WHO reported that dengue outbreaks in Indonesia (E000099) are at level 4. Dengue virus (DENV) infection is a significant cause of acute febrile illness in Indonesia, a DENV-endemic region that has experienced a 700-fold increase in incidence over the past 45 years. As of July 2024, 149,866 confirmed cases of Dengue and 884 deaths had been reported from 465 districts across 38 provinces in Indonesia.
Data published by the Dengue for Community Portal indicates that Taman Mawar-Sendang was a dengue hotspot in Malaysia in June 2025.
The French Department of Mayotte has confirmed 21 cases of Dengue since the beginning of 2025.
On April 9, 2025, the WHO intervened to prevent dengue outbreaks in Myanmar, distributing 4,500 rapid diagnostic test kits to frontline responders and health workers in displacement sites and remote villages.
The Republic of Nauru Ministry of Health has confirmed 379 dengue cases, and two fatalities were recorded on July 30, 2025.
As of June 20, 2025, the Republic of the Philippines reported about 100,000 dengue cases and a case fatality rate of 0.36%. In 2025, the Quezon City Government in the Philippines, through the City Health Department (QCHD), declared a dengue outbreak. From January to July 2025, the City Epidemiology and Surveillance Division of QCHD reported 5,702 dengue cases. In 2025, a total of 390 dengue cases, along with three deaths, were recorded in Antique. In 2024, the Republic of the Philippines Negros Occidental had 6,799 dengue cases and 22 dengue-related deaths in 2024.
The Independent State of Samoa announced on April 17, 2025, that the Ministry of Health is officially declaring a dengue fever outbreak. As of June 2, 2025, the Ministry of Health reported a total of 211 confirmed cases and one reported death. The majority of cases (76%) are from Upolu Island.
According to Sri Lanka's National Dengue Control Unit, 19,901 dengue cases were reported in the country in 2025.
During the first half of the 20th century, there were three island–wide dengue fever outbreaks in Taiwan. After almost forty years of dormancy, a DEN–2 outbreak occurred in 1981. Since 2006, Taiwan has faced dengue fever outbreaks of different scales every year. As of August 2025, a dengue cluster was reported in Kaohsiung's Gushan District.
Dengue Outbreaks in the Eastern Mediterranean Region
Dengue and severe dengue epidemics were first reported in the WHO's Eastern Mediterranean Region in 1998. Since then, outbreaks have occurred in all nine endemic countries: Afghanistan, Djibouti, Egypt, Oman, Pakistan, Saudi Arabia, Somalia, Sudan, and Yemen. On July 17, 2024, the WHO reported 12 autochthonous (local) cases of Dengue documented in Iran. In August 2024, dengue screening was launched at the Iranian border.
Dengue Outbreaks in Europe
In Europe, dengue viruses, transmitted by Aedes albopictus mosquitoes, are primarily associated with infections acquired in endemic countries. Local transmission remains rare, with only sporadic or small-scale outbreaks documented. As of December 2024, the European Centre for Disease Prevention and Control (ECDC) reported over 8,500 dengue-related deaths. In 2024, the ECDC reported locally acquired dengue cases in France, Germany, Italy, and Spain. Over 1,600 DENV infections in Germany occurred exclusively in travellers returning from dengue-endemic countries in 2024. In 2023, 130 locally acquired dengue cases were reported in the European Union and the European Economic Area (EU/EEA). The number of imported dengue cases in Europe increased from 1,572 in 2022 to approximately 4,900 in 2023.
On July 24, 2025, three confirmed cases of dengue fever were recorded in Budrio, Italy. As of July 15, 2025, Italian health authorities reported the first locally acquired case of Dengue of the year in Italy. The Italian National Public Health Authority reported 213 locally acquired dengue cases in 2024 and 82 cases in 2023. In Fano, a small coastal city in the Marche Region, Eurosurveillance reported 138 confirmed and 61 probable cases of DENV-2 as of October 28, 2024. Travel-related dengue cases have reached 472 in 2024. Non-travel-associated dengue cases have been reported in Italy since 2010, with a total of 10 cases.
On July 15, 2025, a second locally acquired case of Dengue was reported this year in Saint-Chamond, Auvergne-Rhône-Alpes, France. In 2024, France reported 82 locally transmitted cases of dengue fever and over 4,600 imported cases. Outbreaks were identified in the Provence-Alpes-Côte d'Azur, Occitanie, and Auvergne-Rhône-Alpes regions. In 2023, France reported nine dengue outbreaks, which resulted in 45 autochthonous infections. In 2022, France reported 65 locally acquired cases of Dengue.
In February 2025, the Autonomous Region of Madeira (Portugal) reported the results of entomological investigations confirming the presence of Dengue mosquitoes captured on Madeira. Two people were infected. The 2012 dengue outbreak affected approximately 1,000 residents.
Spain reported eight locally acquired dengue cases in the Camp de Tarragona area of the Catalonia region in 2024, and the Catalonia region reported three local cases in 2023.
Dengue Outbreaks in India
India has confirmed Dengue outbreaks for decades. In 2024, over 233,000 Dengue cases and 236 deaths were reported. As of 2024, dengue cases in India are on the rise, particularly in the states of Karnataka, Kerala, and Tamil Nadu. They generally peak in October. According to data from the National Centre for Vector-Borne Diseases Control Program, India reported 289,235 dengue cases and 485 related deaths in 2023. In the Democratic Socialist Republic of Sri Lanka, the co-circulation of multiple dengue virus genotypes was reported in October 2024 to be associated with an increase in cases.
Dengue United Kingdom 2025
The UK Health Security Agency (UKHSA) states that local dengue fever does not occur in the United Kingdom; however, it can be acquired by traveling to dengue-endemic areas. In 2024, 904 dengue cases were reported in returning travellers across England, Wales, and Northern Ireland, up from 631 in 2023. The most significant proportion of English cases (349) was reported in London. This data represents a 201% increase compared to the same period in 2023, which saw 157 cases. In 2024, India remained the most reported travel destination, with 179 cases, a 23% increase compared to 2023.
Dengue Virus-Carrying Mosquito
Mosquito bites cause more human suffering than any other organism. The spread of Dengue throughout the world can be directly attributed to the proliferation and adaptation of these mosquitoes. In the U.S., there are 176 species. A recent study published by the Royal Society indicates that dengue-carrying mosquitoes are expanding their range by an average of 6.5 meters of elevation and have moved polewards by 4.7 km annually.
Dengue Virus Infection Testing
The U.S. Centers for Disease Control and Prevention (CDC) published a Health Update (CDCHAN-00523) on March 18, 2025, highlighting the ongoing risk of Dengue virus infections and updates to testing recommendations in the United States. People with suspected Dengue virus infection should be tested with a real-time PCR, NS1 antigen test, or an IgM enzyme-linked immunosorbent assay antibody test at commercial labs or public health clinics.
Dengue Disease
Dengue is a disease caused by a virus transmitted through the bites of infected mosquitoes. It can take up to two weeks to develop, but the illness generally lasts less than a week. Without treatment, severe Dengue can become fatal. New research has identified pre-existing anti-DENV IgG antibodies as the cause of the increased duration of Dengue upon second exposure.
Severe Dengue
Approximately 5% of Dengue cases can progress rapidly to Severe Dengue, which may involve hypovolemic shock, gastrointestinal or vaginal bleeding requiring transfusion, and end-organ impairment. Furthermore, women infected with Dengue during pregnancy can pass the virus to their fetuses. Promptly initiating intensive supportive therapy can reduce the risk of death among patients with severe Dengue. The extent and duration of viremia are often correlated with the severity of clinical disease. A study published in October 2024 concluded that secondary dengue infections with different dengue virus serotypes have been linked to an increased risk of Severe Dengue after two years.
Dengue Infections Cause Cardiovascular Complications
Published on April 18, 2025, this review discusses the cardiovascular manifestations of Dengue and their management, explores the proposed pathogenesis, and concludes with a discussion of potential future research directions.
Dengue Viruses
There are four Dengue Viruses. A study published in October 2024 concluded that the co-circulation of multiple genotypes is associated with an increase in severe cases, highlighting the importance of continuous surveillance.
Dengue Virus Blood Transfusion-Transmission
Emerging evidence published in November 2024 suggests a potentially concerning route of blood transfusion-transmitted dengue virus (TT-DENV), which poses a critical threat, especially in endemic countries like Brazil. In May 2024, a RESEARCH ARTICLE found that dengue virus transmission was a risk in blood donation in Thailand. In March 2016, Transfusion-Transmitted Dengue and Associated Clinical Symptoms During the 2012 Epidemic in Brazil was published.
Dengue Infection Immune-Mediated Enhancement
In this study, published on October 31, 2024, researchers demonstrate that the expression of a DENV-specific B cell receptor (BCR) renders cells highly susceptible to DENV infection, with the infection-enhancing activity of the membrane-restricted BCR correlating with the antibody-dependent enhancement (ADE) potential of the IgG version of the antibody. In addition, they observed that the frequency of DENV-infectible B cells increases in previously flavivirus-naïve volunteers after a primary DENV infection. These findings suggest that BCR-dependent infection of B cells is a novel mechanism for immune-mediated enhancement of DENV infection. This observation indicates that BCR-dependent infection of DENV-specific B cells may be a complementary mechanism for immune-mediated enhancement of DENV infection, expanding upon existing models of antibody-dependent enhancement.
Dengue Outbreak Discrepancy Research
In October 2024, a study published by The Lancet Infectious Diseases provided novel insights into serotype-specific epidemiological patterns and disease outcomes of primadengue virus (rDENV)ENV infections by revealing the hidden contribution of inapparent infections. This indicates that case surveillance skews the perceived epidemiological footprint of Dengue's four viruses. On October 25, 2024, these researchers wrote, 'While inapparent infections are often overlooked and do not require immediate medical attention, we have assumed that they account for up to 88% of all dengue virus (DENV) transmission events. A study published in May 2024 revealed substantial discrepancies between estimates and reported numbers of dengue cases. A study published in the journal Nature on January 20 revealed variations in antibody composition that contribute to the miscounting of primary and secondary infections.
Dengue Virus in Pregnant Women
The CDC confirms that a pregnant woman already infected with Dengue can pass the virus to her child during pregnancy, and there has been one documented report of Dengue spread through breast milk. A study published in the American Economic Journal: Applied Economics in April 2024 confirmed robust evidence for the adverse effect of dengue infections on birth weight and documented increases in children's hospitalizations and medical expenditures for up to three years after birth.
Dengue and Zika Virus
A study published in the journal Science Translational Medicine on May 29, 2024, found that primary ZIKV infection increased the risk of disease caused by DENV3 and DENV4 but not DENV1. This finding was also observed for tertiary infections in individuals previously infected with DENV and ZIKV, but not in those previously infected with ZI alone.
Dengue Vaccines
Information on dengue vaccines (Qdenga), vaccine candidates, and clinical trials in 2025 can be found at Vax-Before-Travel.