Search API
The World Health Organization (WHO) today published an update on the Nipah virus (NiV) outbreak in India. Since 2018, Kerala has experienced nine outbreaks of the Nipah virus, which is part of a pattern of recurring spillovers.
As of August 6, 2025, Kerala State health officials have reported four cases to the WHO since mid-May, two of which have been fatal.
Of the latest four patients in India's southwest area, two are from Malappuram district, where previous cases had been reported. The other two are from Palakkad district, which had not seen any earlier cases.
According to the WHO, NiV infection is a bat-borne zoonotic disease transmitted to humans through infected animals, or food contaminated with saliva, urine, and excreta of infected animals.
Since 1998, NiV outbreaks have been reported in Bangladesh, India, Malaysia, the Philippines, and Singapore.
Currently, the risk of international disease spread is considered low, as there is no evidence of human-to-human transmission of NiV internationally in this event.
The WHO wrote that, with no licensed vaccine or treatment available, public health efforts should focus on raising awareness of risk factors and promoting preventive measures to reduce exposure to the virus, and on early case detection supported by adequate intensive supportive care.
In 2023, the Coalition for Epidemic Preparedness Innovations invested $$100 million in four Nipah vaccine candidates: Auro Vacc, PATH, Public Health Vaccines, the University of Tokyo, and the University of Oxford.
Recently, the U.S. government announced a project in 2025 that would support the development of a Nipah monoclonal antibody (MBP1F5), currently undergoing Phase 1 testing in India and Bangladesh.
When Ebola and Marburg outbreaks have occurred over the decades, diagnosing cases has been a significant challenge for healthcare workers.
To address this essential need, Aptitude Medical Systems announced its second major partnership with the Biomedical Advanced Research and Development Authority (BARDA), with $9 million in funding to develop the Metrix Filovirus Panel.
This collaboration leverages Aptitude's next-generation molecular diagnostics platform, Metrix®, which has been advanced through a prior BARDA partnership valued at up to $61.9 million.
This rapid next-generation molecular diagnostic device aims to detect and differentiate Ebolavirus and Marburgvirus species.
"Point-of-care diagnostics are essential for effectively addressing outbreaks of high-consequence pathogens like Ebolavirus and Marburgvirus species," added JP Wang, PhD, CTO, President, and Executive Chairman of Aptitude, in a press release on July 28, 2025.
It is a small, portable platform, making it appropriate for use in remote and more traditional point-of-care settings, generating results in 30 minutes or less from venous or fingerstick blood samples.
As of July 29, 2025, there are no active Ebola and Marburg outbreaks in Africa, and Ebola vaccines and antibody therapies have been approved for use by various countries.

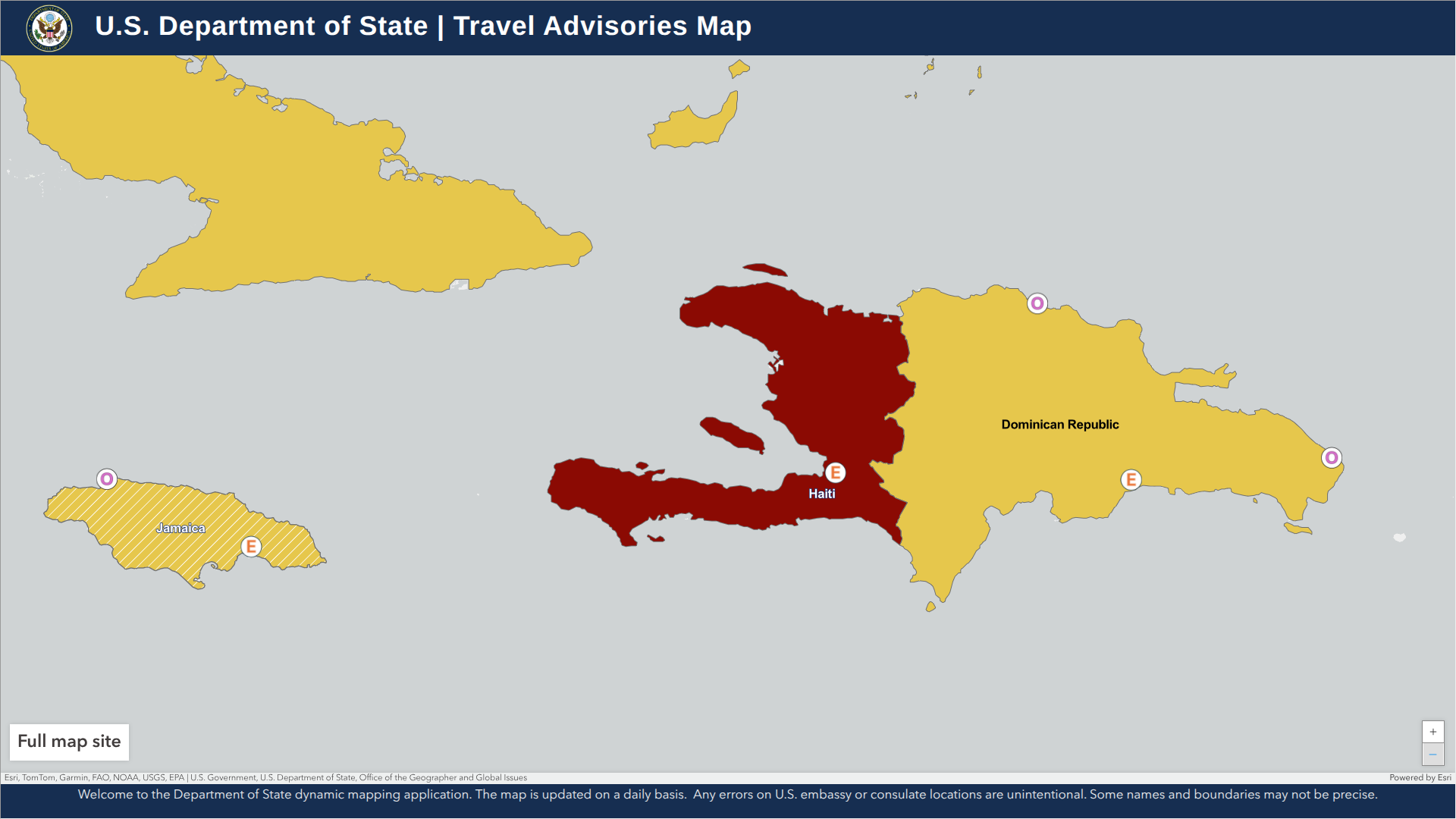
As the prolonged civil unrest continues to challenge the government of Haiti, the western part of the island of Hispaniola remains at risk for international visitors.
Haiti has been under a State of Emergency since March 2024.
According to the U.S. Department of State's updated Level 4: Do Not Travel advisory, as of July 15, 2025, civil unrest continues, and access to healthcare services in Haiti remains very limited for both residents and visitors.
Furthermore, the U.S. government has limited ability to assist U.S. citizens in Haiti. Local police and first responders often lack sufficient resources.
U.S. government employees working in Haiti must obtain special permission to travel outside the embassy compound due to security risks, and Embassy personnel are prohibited from traveling on foot in the capital city of Port-au-Prince.
Additionally, do not cross the land border between Haiti and the Dominican Republic, as the roads from major Haitian cities to the border pose significant dangers.
In addition to the U.S., the UK's Foreign, Commonwealth & Development Office has issued similar advice for Haiti in 2025. If you choose to travel, obtain appropriate travel insurance that covers your itinerary, planned activities, and expenses in the event of an emergency.
From a health perspective, the U.S. CDC recommends that international travelers planning to visit Haiti speak with a travel vaccine consultant at least one month before departure to obtain the necessary vaccinations and medical supplies.
The CDC highlights travel vaccines, including those for chikungunya, cholera, and rabies, before visiting Haiti.
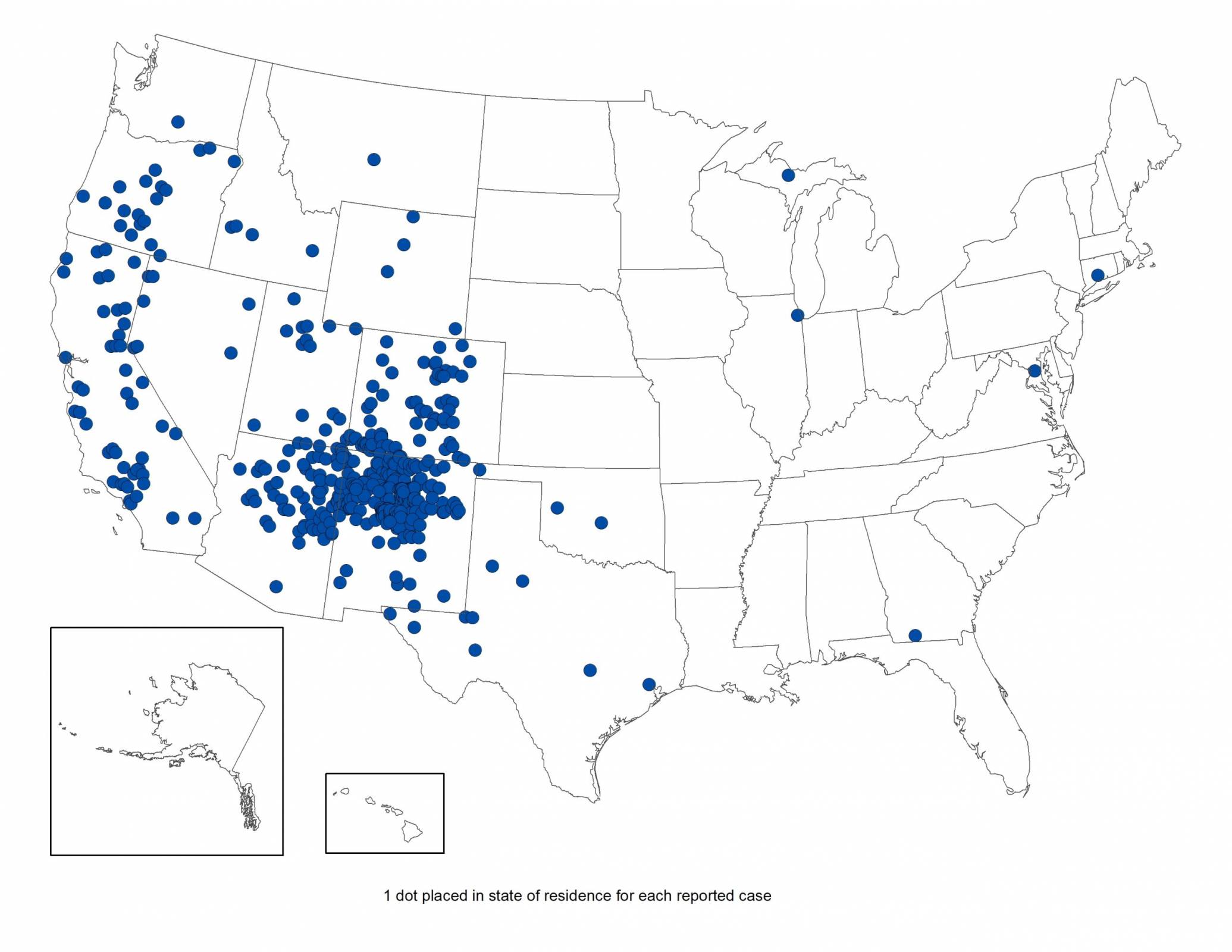
Coconino County Health and Human Services officials recently confirmed a resident died from pneumonic plague, a severe lung infection caused by the Yersinia pestis bacterium.
As of July 11, 2025, this is the first recorded death from Pneumonic plague in Coconino County since 2007, when an individual had an interaction with a dead animal infected with the disease.
The new death is not related to a recent report of a prairie dog die-off in the Townsend Winona area, northeast of Flagstaff, Arizona.
“Our hearts go out to the family and friends of the deceased,” said Coconino County Board of Supervisors Chair Patrice Horstman in a press release.
“Out of respect for the family, no additional information about the death will be released.”
According to the U.S. Centers for Disease Control and Prevention, an average of seven human plague cases are reported each year in the United States. The last urban plague epidemic in the United States occurred in Los Angeles from 1924 through 1925.
The CDC states that the risk to the local public from exposure to plague remains low.
More recent plague epidemics have occurred in Africa, Asia, and South America.
The bacterium that causes plague, Yersinia pestis, can be transmitted to humans from the bite of an infected flea or through contact with an infected animal. According to health officials, the risk of human-to-human transmission is very low. The last reported occurrence of human-to-human transmission in the U.S. was reported in 1924.
Without a vaccine to prevent plauge epidemics, the U.S. Department of Defense (DoD) continues to fund development efforts.
Dynavax Technologies Corporation confirmed in February 2025 that it continues developing a plague vaccine candidate (rF1V) with the CpG 1018® adjuvant in collaboration with, and fully funded by, the DoD.
Dynavax and the DoD executed a new agreement for approximately $30 million through the first half of 2027 to support additional clinical and manufacturing activities, including a Phase 2 clinical trial expected to initiate in the third quarter of 2025.
The World Health Organization (WHO) recently confirmed that the spread of the poliovirus remained a global Public Health Emergency of International Concern.
To address the under-vaccinated population, two announcements indicate a path to reduce the detections of poliovirus and minimize the number of polio outbreaks.
On July 10, 2025, PharmaJet® announced that it has signed a Memorandum of Understanding to explore the integration of needle-free delivery of inactivated polio vaccine into Egypt’s routine immunization program. The agreement includes provisions for distribution, technology transfer, manufacturing, and the development of new pharmaceutical products, as well as collaboration to increase needle-free access within Egypt and the region.
Seperately, the Government of Japan, through the Japan International Cooperation Agency, has provided UNICEF with $5 million for a renewed partnership to eradicate polio and strengthen routine immunization across all 34 provinces of Afghanistan. This 12-month initiative aims to reach over 13 million children with lifesaving polio vaccines, supporting both routine vaccination and national polio campaigns.
Previously, to alert international travelers of the expanded health risk in 41 countries, the U.S. Centers for Disease Control and Prevention reissued a Global Polio Alert—Level 2, Travel Health Notice on June 16, 2025, regarding polio outbreaks and poliovirus detections.
As of July 12, 2025, the CDC recommends that travelers complete their routine polio vaccination series. Some people may also need a booster dose before visiting areas with outbreaks, such as Afghanistan and Germany.
In the United States, polio vaccines are available at travel clinics and pharmacies.