$30 Million Continues to Fund DoD Plague Vaccine Candidate
Dynavax Technologies Corporation today confirmed it continues developing a plague (rF1V) vaccine candidate adjuvanted with CpG 1018® in collaboration with, and fully funded by, the U.S. Department of Defense (DoD).
As of February 20, 2025, based on the results from a randomized, active-controlled Phase 2 clinical trial, Dynavax and the DoD executed a new agreement for approximately $30 million through the first half of 2027 to support additional clinical and manufacturing activities, including a Phase 2 clinical trial expected to initiate in the third quarter of 2025.
As previously announced, Dynavax and the DOD executed an earlier agreement providing approximately $22 million in funding to develop the rF1V vaccine.
According to the U.S. CDC, Plague is a potentially deadly infectious disease caused by bacteria found in fleas and rodents or by handling an infected animal. It is caused by the bacterium Yersinia pestis. It is possible that Pneumonic plague bacteria could be released intentionally in a biological attack to sicken people.
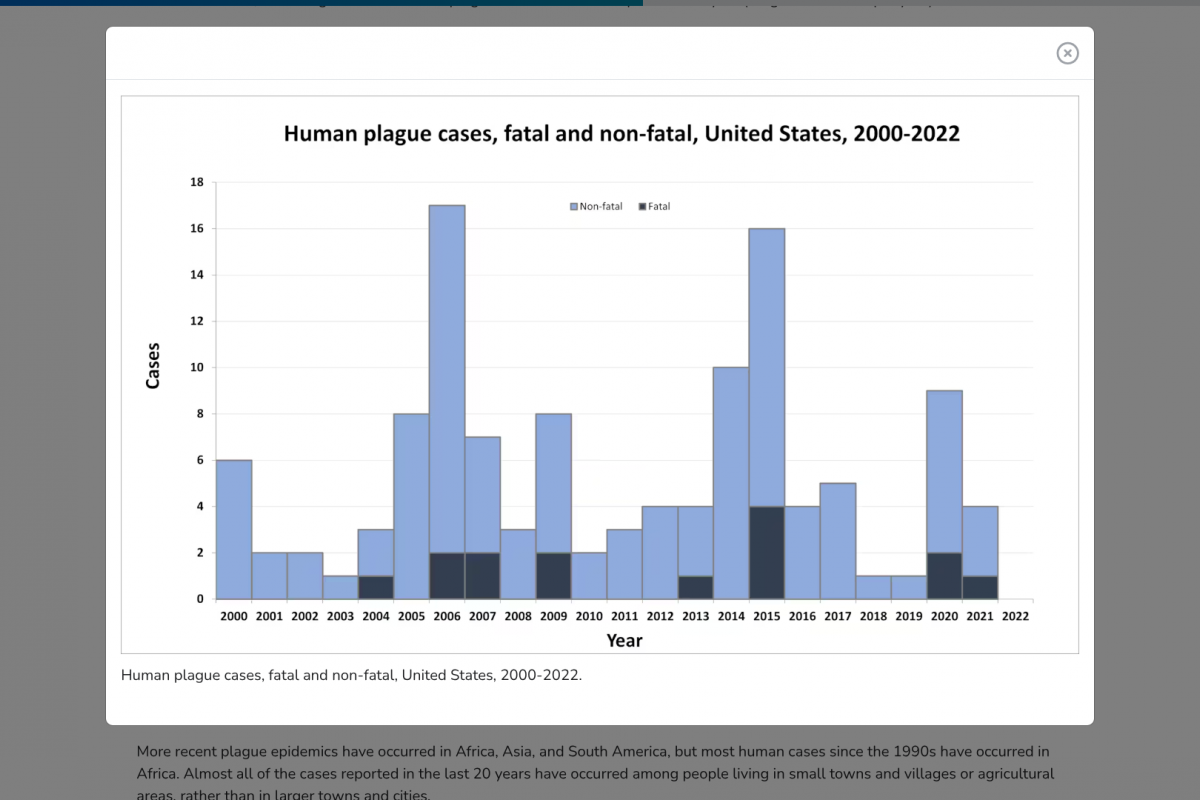
Since the mid–20th century, plagues in the United States have typically occurred in the rural West. The CDC says cases in the eastern United States are among people who traveled from the west or have laboratory exposure.
More recent plague epidemics have occurred in Africa, Asia, and South America.
Our Trust Standards: Medical Advisory Committee
