Search API
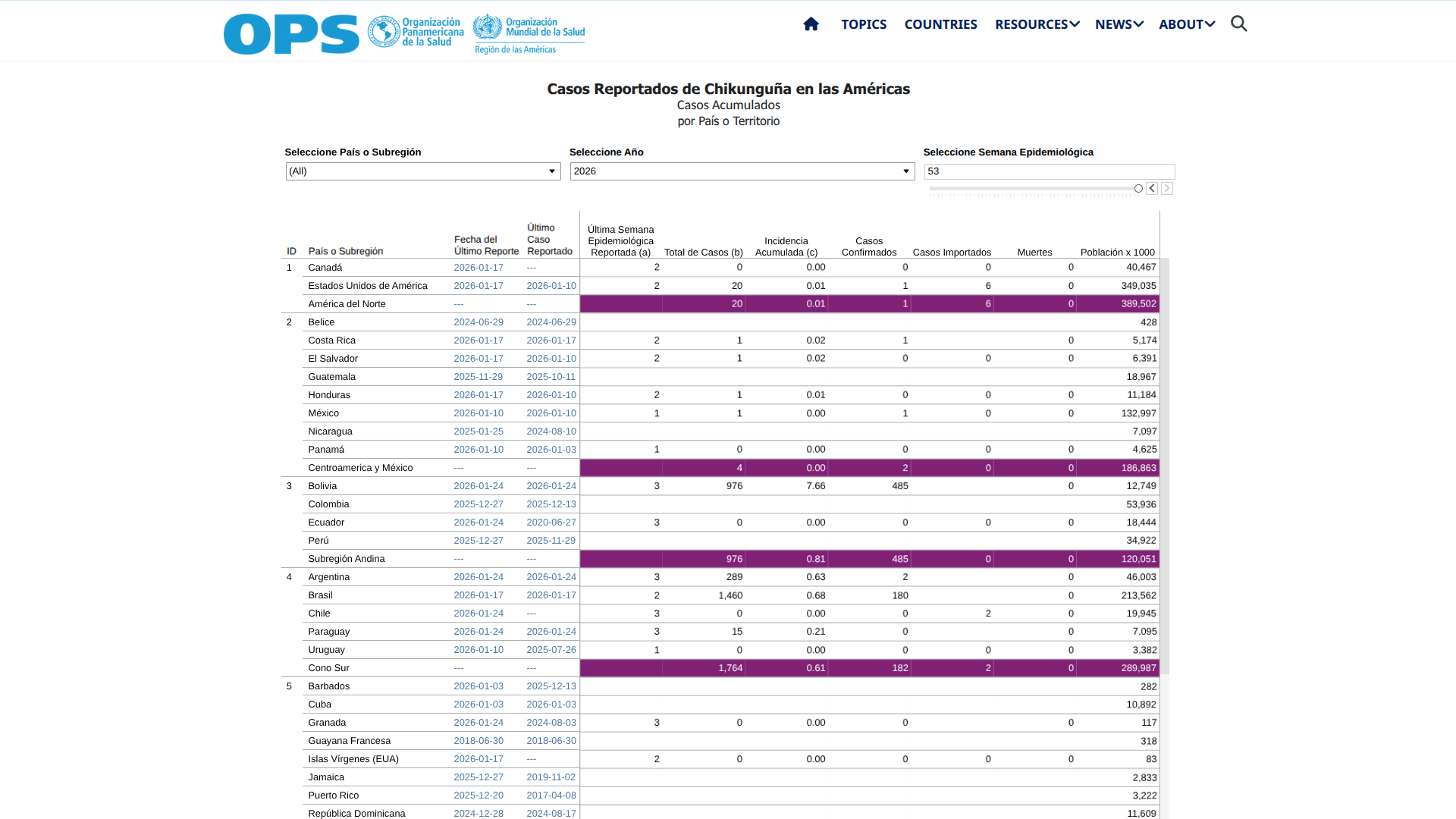
The Florida Department of Health (DOH) today announced updated surveillance data on chikungunya fever cases in the state.
As of February 4, 2026, the data emphasize the ongoing risks associated with travel to chikungunya-endemic areas, particularly Cuba, as well as the rare but noteworthy occurrence of local transmission in Florida.
As of the latest reporting period (#4) in 2026, nine cases have been confirmed among individuals with travel history to chikungunya-endemic areas within two weeks before onset, all linked to travel to Cuba.
And one case of locally acquired chikungunya fever was reported in Miami-Dade County, with symptom onset in December 2025.
In 2025, a total of 350 chikungunya cases were reported among individuals who had recently traveled to chikungunya-endemic areas. These cases were distributed across multiple Florida counties, with Miami-Dade County reporting the highest number at 229.
Chikungunya fever is a viral illness primarily transmitted by Aedes mosquitoes. Symptoms include high fever, severe joint pain, muscle pain, headache, nausea, fatigue, and rash. Most individuals recover completely; however, joint pain can be debilitating and may persist for months in some cases. There is no specific antiviral treatment, but supportive care can help relieve symptoms.
The DOH and the U.S. CDC advise travelers to endemic areas to consult local healthcare clinics about preventive strategies, including vaccination options. In Florida and most states, U.S. FDA-approved chikungunya vaccines are available in 2026.
In the past year, the World Health Organization (WHO) reported over 600,000 cases of cholera or acute watery diarrhea and nearly 7,600 deaths across 33 countries.
However, these figures are likely underreported, as cholera cases often go unrecorded.
Today, Gavi, the Vaccine Alliance, UNICEF, and the WHO announced that the global supply of oral cholera vaccine (OCV) has increased sufficiently to resume life-saving preventive vaccination campaigns for the first time in over three years.
A first allocation of 20 million doses is being deployed for preventive campaigns.
Of these, 3.6 million doses were delivered to Mozambique; 6.1 million to the Democratic Republic of the Congo, which is also experiencing significant outbreaks; and 10.3 million doses are planned for delivery to Bangladesh.
"Global vaccine shortages forced us into a cycle of reacting to cholera outbreaks instead of preventing them. We are now in a stronger position to break that cycle. I thank EUBiologics, currently the only manufacturer producing cholera vaccines at the scale needed for mass vaccination campaigns, for its efforts, and urge others to enter this vital space. These vaccines will save lives," said Dr Tedros Adhanom Ghebreyesus, WHO Director-General, in a press release on February 4, 2026.
Over the last few years, the annual global supply of OCV has doubled from 35 million doses in 2022 to nearly 70 million doses in 2025. The doses are being financed by Gavi and procured and delivered to countries by UNICEF.
"The multi-year surge in cholera cases and resulting unprecedented demand for vaccines were stark reminders that sustainable, accessible vaccine supply is a global public good – and the world cannot afford complacency," added Dr Sania Nishtar, CEO of Gavi, the Vaccine Alliance.
"For the first time in years, this increase in vaccines will allow us to prevent large-scale cholera emergencies better," said Catherine Russell, UNICEF Executive Director. "Resuming preventive cholera vaccination will protect children and help stop this highly contagious disease in its tracks. But it must go hand in hand with other efforts, including better access to safe water and basic sanitation."
While global vaccine supply steadily improves, the one-dose strategy will remain the standard for outbreak responses, with the use of two doses considered on a case-by-case basis.
Cholera spreads through contaminated food and water, causing severe diarrhoea and dehydration. It can lead to death if it is not treated quickly. It is found in places without safe water and sanitation, mainly in localities affected by conflict and poverty.
The WHO says vaccination is only one aspect of cholera prevention and response. Long-term investments in safe water, sanitation, and hygiene infrastructure, alongside disease surveillance, rapid treatment, and community engagement, remain essential to prevent outbreaks from starting and spreading, and to reduce deaths in the long term.
In the United States, OCVs are offered at travel vaccine clinics, located in every state.
The Republic of Paraguay's Ministry of Public Health and Social Welfare announced today that the Dengue vaccination strategy, initially targeted at children in priority municipalities, is now being expanded to include individuals aged 39 and younger.
Announced on February 3, 2026, this decision is based on an epidemiological analysis conducted over recent years, which indicates a significant burden of Dengue not only among children but also among adolescents and young adults.
Between 2019 and 2024, the 20 to 39-year-old age group accounted for more than 108,000 Dengue cases in this South American country, reflecting one of the highest incidence rates recorded.
These findings support the extension of the second-generation, two-dose, Qdenga (TAK-003) vaccine, which protects against all four serotypes of the Dengue virus.
Paraguay says vaccination is a critical component of a comprehensive strategy that includes epidemiological surveillance and preventive measures to mitigate the impact of Dengue in the country.
Paraguay's neighbor, Brazil, recently announced it would focus on a newly approved third-generation, single-dose Butantan-DV vaccine for its residents.
Currently, Dengue vaccines are unavailable in the continental United States.
As of February 2026, the U.S. Food and Drug Administration (FDA) has not yet approved any vaccine for the herpes simplex virus (HSV). However, several candidates are currently undergoing clinical trials.
These efforts aim to tackle the global impact of HSV-1 and HSV-2 infections, which cause oral and genital herpes in millions of people annually.
Unfortunately, the FDA states that there is currently no cure for HSV infections.
However, antiviral medications can help shorten the duration of herpes outbreaks. A significant advancement in HSV treatment is currently attracting attention.
AiCuris Anti-infective Cures AG is set to present detailed results from its pivotal Phase 3 clinical trial of pritelivir as a late-breaking oral presentation at the Tandem Meetings 2026, held February 4-7 in Salt Lake City, Utah.
Pritelivir, a novel oral helicase-primase inhibitor, targets both HSV-1 and HSV-2 by blocking viral DNA synthesis through a mechanism distinct from traditional nucleoside analogs like acyclovir or valacyclovir.
This unique mode of action makes it effective against strains resistant to current standard therapies, addressing a critical unmet need—particularly in immunocompromised patients, where resistant or refractory HSV infections can lead to severe complications and limited treatment options.
The PRIOH-1 trial focused on immunocompromised individuals with acyclovir-refractory (and sometimes foscarnet-resistant) mucocutaneous HSV infections. In October 2025, AiCuris announced that pritelivir met its primary endpoint, demonstrating statistically significant superiority in lesion healing compared to the investigator's choice of standard-of-care treatments (such as foscarnet, cidofovir, or topical options).
Superiority was shown for treatment up to 28 days (p=0.0047), with even stronger results extending to 42 days (p<0.0001).
Earlier Phase 2 data supported a favorable safety profile, and pritelivir has held an FDA Breakthrough Therapy designation for this indication since 2020. AiCuris expects to file for FDA marketing authorization in 2026, potentially offering the first new HSV therapy in decades that is active against resistant strains.
Full abstracts will be released on February 4, 2026, and will be available via the conference website here.
This development highlights progress in managing HSV complications in high-risk groups, even as preventive vaccines remain further on the horizon. Ongoing research and presentations like this at major conferences continue to drive hope for improved options against this widespread virus.

Three months after Hurricane Melissa made landfall in Jamaica on October 28, 2025, as the strongest Category 5 storm ever to hit the Caribbean island, the country's health system is still in the process of recovery.
The aftermath has resulted in widespread damage and ongoing public health risks.
In this interview, published on February 2, 2026, Ian Stein, the Pan American Health Organization/World Health Organization country representative in Jamaica, shares insights into the realities of recovery, lessons learned, and what success looks like in a complex emergency.
As of 2026, the U.S. CDC website states that, in the wake of Hurricane Melissa, travel by land may be dangerous in flood zones, and the healthcare infrastructure has been damaged in these areas.
There may be an increased risk of waterborne (including leptospirosis), vector-borne, and fungal diseases. And avoid contact with floodwater.
Furthermore, before visiting Jamaica in 2026, check the list of recommended vaccines and medications and see your travel health provider at least a month before your trip to get any you may need.
From a security perspective, the U.S. Department of State lowered its advisory for Jamaica to Level 2: Exercise Increased Caution on January 17, 2026, citing crime, health, and natural-disaster risks.
The State Department advisory clarifies that some areas have an increased risk. The U.S. advises against traveling to these areas for any reason.
The Republic of Costa Rica's tourism sector has started the 2026 winter vacation season on a positive note, with reports of strong international arrivals.
By early February 2026, Costa Rican tourism authorities had noted bustling activity at the country's main airports: Juan Santamaría International Airport in the Central Valley and Daniel Oduber Quirós International Airport.
Like past seasons, snowbirds are attracted to Costa Rica's sunny beaches, volcanoes, rainforests, and eco-tourism options.
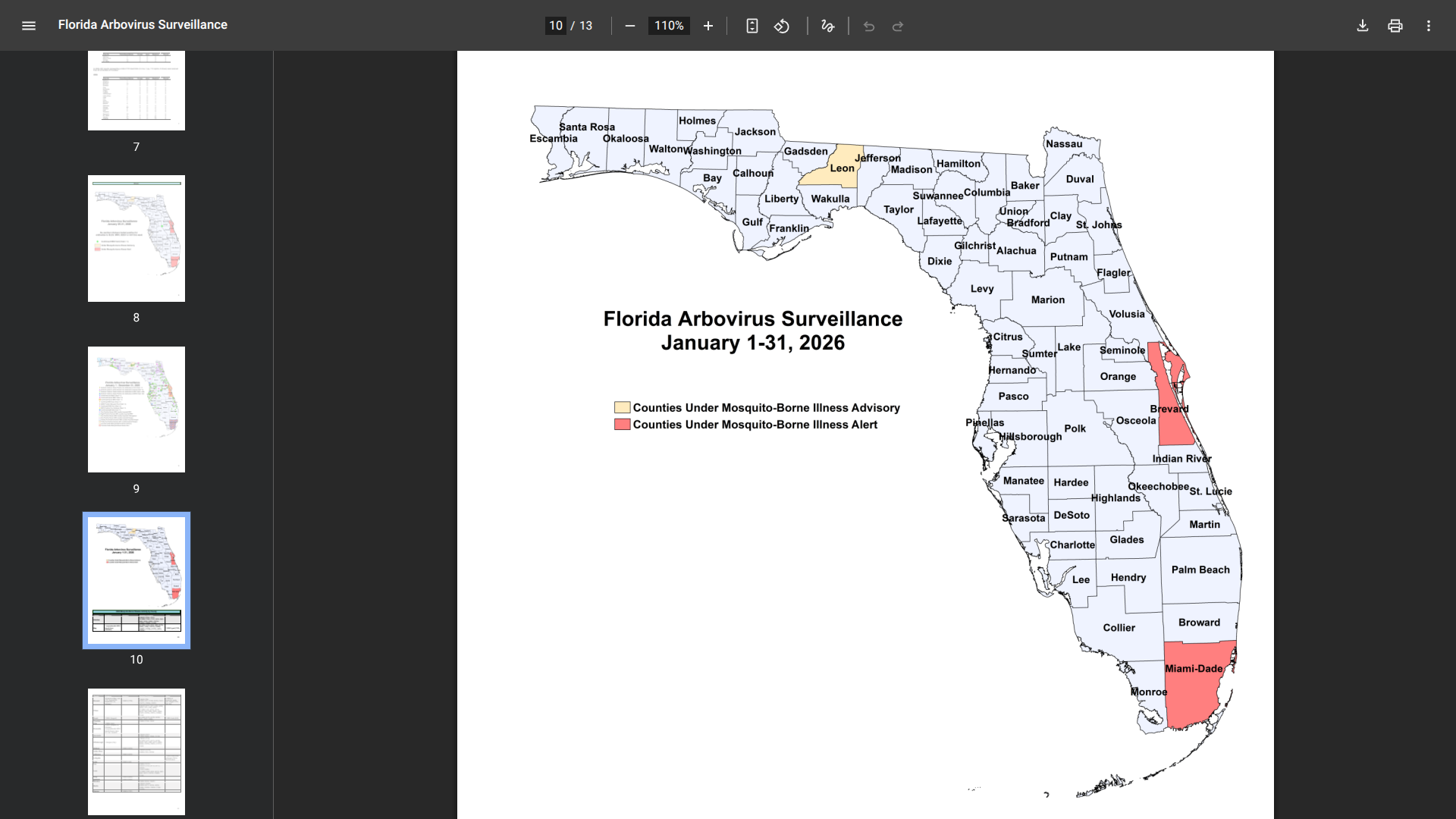
However, amidst this promising tourism rebound, health authorities have issued a warning regarding mosquito-borne diseases.
On January 29, 2026, the Ministry of Health confirmed a second case of Chikungunya in a resident of Esparza, Puntarenas province—the same canton where the first case was confirmed earlier in January.
Based on the onset of symptoms, the second patient may have been infected around the same time as the initial case.
Puntarenas is located in the western part of the country, covering most of Costa Rica's Pacific Ocean coast. And Esparza is situated between the mouths of the Río Barranca and the Río Jesús María rivers.
Vector control efforts in Esparza are ramping up, with teams having already fumigated 10,210 homes and buildings, applied insecticides using LECO sprayers and tractor-mounted foggers, and conducted thorough searches for individuals exhibiting fever symptoms.
These measures will continue over the next few weeks to prevent further spread of the virus.
Last year, eight cases of Chikungunya were reported, a marked improvement from 2024, when over 400 cases were reported.
The Ministry of Health has reminded the public that Chikungunya is transmitted by the same Aedes mosquitoes that spread Dengue and Zika.
Symptoms typically include fever, severe joint pain, muscle pain, headache, nausea, fatigue, and rash. While most cases are mild and resolve within weeks, joint pain can persist.
While no vaccines are required for entry into Costa Rica when traveling from the United States, chikungunya vaccines are available at travel vaccination clinics before departure abroad.
Travelers are advised to stay informed about health recommendations from the Ministry of Health, the U.S. CDC, or reliable sources such as vax-before-travel.com.
Chikungunya is a viral disease spread through the bites of infected mosquitoes. Since 2013, the virus has been detected in the Americas, and it has rapidly spread throughout Brazil.
Brazil has reported the highest number of chikungunya cases worldwide, including 246 related fatalities in 2024.
As of early February 2026, Brazil has reported 1,480 suspected and 180 confirmed cases of chikungunya.
In response to this serious public health issue, Brazil is taking additional measures to secure access to chikungunya vaccines.
Valneva SE today announced the launch of a Pilot Vaccination Strategy (PVS) in Brazil in collaboration with Instituto Butantan, one of the world's largest biomedical research centers. The companies initially signed a technology transfer agreement in January 2021.
This new PVS program will utilize Valneva's single-dose chikungunya vaccine, IXCHIQ®.
The goal of this program is to support post-marketing commitment studies that will assess the effectiveness and safety of IXCHIQ® in real-world conditions, providing valuable data from a large population.
Esper Kallas, M.D., Ph.D., The Director of the Instituto Butantan outlined the path toward the PVS program on February 3, 2026, stating: "With ongoing engagement from the Ministry of Health, regional health secretaries, and ANVISA, I am confident that we have established a program that is both robust and compliant with regulatory standards."
"These combined efforts are expected to ensure timely access to vaccination and alleviate the significant public health burden associated with this arboviral disease."
This initiative follows an announcement in January 2026 by Eurofarma. This Brazilian pharmaceutical company stated that it had acquired the rights to sell and distribute Bavarian Nordic's chikungunya vaccine, CHIKV VLP (VIMKUNYA®), in Brazil.
These actions in Brazil may help the state of Florida reduce the number of travel-related chikungunya cases. As of December 2025, 328 travel-related chikungunya cases have been confirmed, many in Miami-Dade County.
Furthermore, 14 cases of chikungunya fever were reported in 2026 among persons who had traveled internationally, with one local case in southeastern Florida.