Search API
Over the past two decades, coronaviruses (CoV) have caused three significant disease outbreaks: severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and coronavirus disease 2019.
Diseases such as MERS continue to cause severe outcomes. There have been a total of 19 MERS-CoV cases reported worldwide since the beginning of 2025, including four fatalities.
To address these diseases with a single vaccine, a candidate designed to provide broad protection against coronaviruses has entered clinical testing.
The vaccine, named GBP511, is built on a self-assembling nanoparticle technology developed by researchers at UW Medicine and the Institute for Protein Design. The South Korean pharmaceutical company SK bioscience is conducting human clinical trials for this vaccine.
GBP511 builds on technology validated through SKYCovione, a vaccine that became the world's first computer-designed medicine to achieve regulatory approval.
In preclinical studies, GBP511 has shown the ability to protect animals from related viruses not directly targeted by the vaccine.
"The beauty of this approach is that by presenting the immune system with multiple related antigens at once, we can train it to recognize features that are conserved across the entire sarbecovirus family," explained David Veesler, a professor of biochemistry at UW Medicine and a Howard Hughes Medical Institute Investigator, who led the preclinical studies, in a press release on February 3, 2026.
He added, "That's exactly what you need to prepare for unpredictable future threats."
The international Phase 1/2 trial began enrolling participants in January 2026 and will assess safety and immune responses in approximately 368 healthy adults in Perth, Western Australia. The study's results are expected by 2028.
The Coalition for Epidemic Preparedness Innovations has supported the GBP511 program with approximately $65 million in funding.

The Ministry of Health and Family Welfare of Bangladesh, in collaboration with the World Health Organization (WHO), announced today a laboratory-confirmed case of Nipah virus (NiV) infection in the Rajshahi Division of northwestern Bangladesh.
As of February 3, 2026, a middle-aged woman from Naogaon District had been experiencing symptoms including fever, headache, muscle cramps, loss of appetite, weakness, and vomiting since late January 2026, when the NiV infection was confirmed. The patient had no travel history outside the area but reported repeatedly consuming raw date palm sap in early January 2026, a known transmission route for the virus due to contamination from fruit bats.
On February 6, 2026, the WHO stated that there are currently no specific treatments or vaccines approved for Nipah, making prevention through public awareness and good hygiene practices essential.
Fortunately, NiV vaccine candidates are being tested in clinical research in 2026.
Since 1998, NiV outbreaks across Bangladesh, India, Malaysia, the Philippines, and Singapore experienced very high fatality rates, ranging from 40% to 75%.
Furthermore, the WHO assessed the overall public health risk posed by this NiV event as low at the national, regional, and global levels. The risk of international disease spread is also considered low, and no travel or trade restrictions are recommended.
An unexpected measles outbreak at Ave Maria University in Collier County, Florida, has intensified, with at least 20 students now confirmed to have contracted the highly contagious viral infection.
As of February 6, 2026, three of these Florida students have been hospitalized due to complications from the illness.
Located northeast of Naples, the school officials stated on their website that they continue to provide care for all students, regardless of test results, and are closely monitoring the situation. In addition to the Padre Pio Campus Health Clinic, a second on-campus clinic has been established to support routine student care.
This outbreak represents the first significant measles activity in Collier County in nearly a decade, as there have been no reported cases during that time.
Alarmingly, university officials have noted that approximately 98% of the student population is vaccinated against measles, which exceeds the U.S. CDC recommendations.
The CDC reports data from 2025-2026, indicating that about 3% to 4% of total reported breakthrough measles cases in the U.S. occurred among people who had received 2 doses.
According to the Florida Department of Health (DOH), there were around 10 confirmed measles cases in southeast Florida (Broward County) in early 2024.
As of early February, the Florida DOH in Collier County is providing additional resources to monitor for suspected measles cases and to minimize further transmission.
Unfortunately, in a potentially related matter, further north in Florida, the administration of St. Petersburg Catholic High School confirmed on Friday, February 4, 2026, that a student has a confirmed case of measles.
The DOH says measles vaccination services are offered at public and commercial locations throughout Florida.
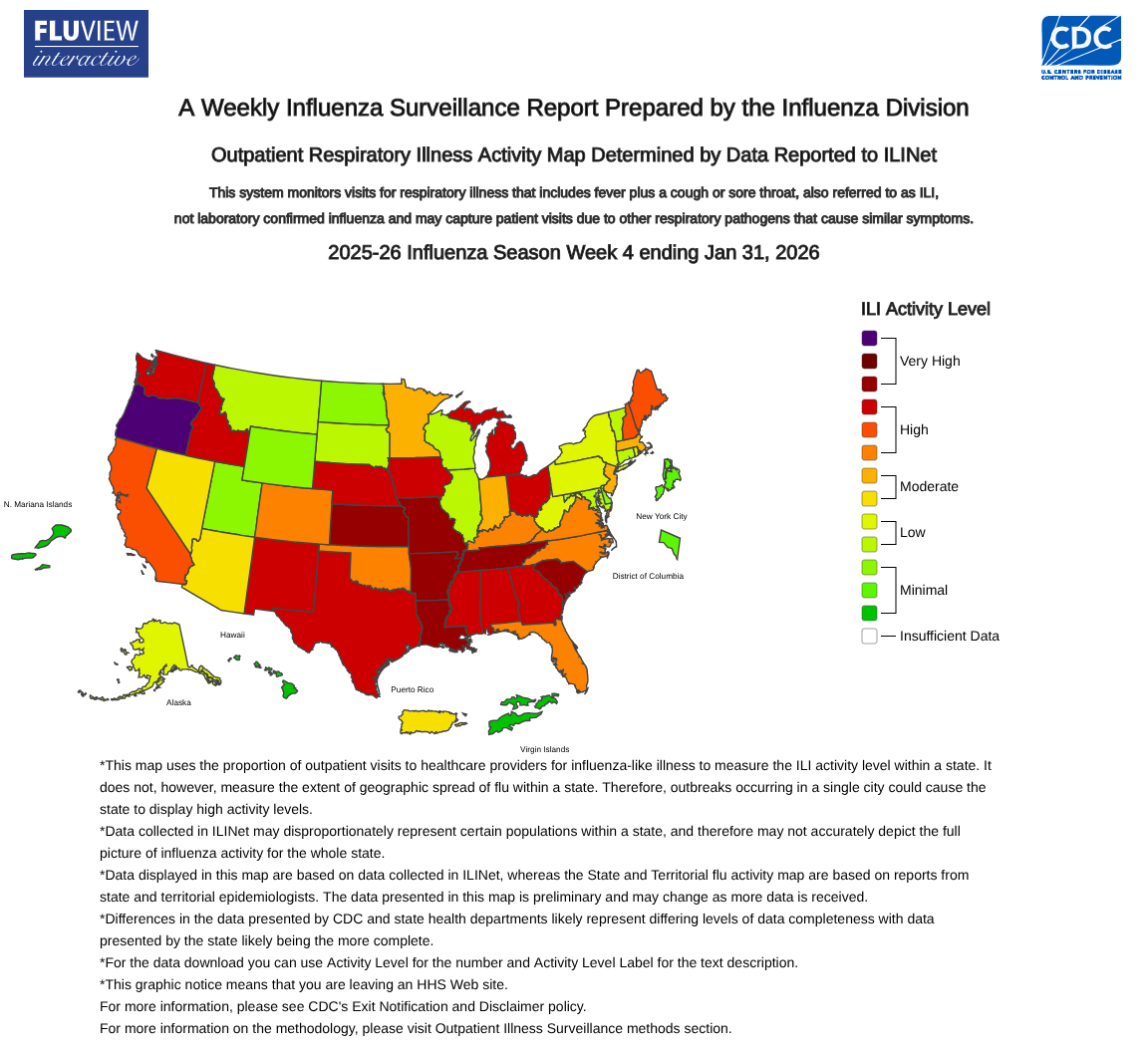
The U.S. Centers for Disease Control and Prevention (CDC) is continuing to monitor elevated levels of seasonal influenza activity across the nation, as detailed in the FluView surveillance report #4.
The CDC's in-season severity assessment framework classified the 2025-2026 flu season as moderate across all ages.
As of February 6, 2026, the CDC reports that influenza activity remains high nationally, although some areas are seeing stabilization or a decline in flu cases.
Most regions are reporting stable or decreasing overall flu activity.
However, influenza activity is still on the rise, with high infection rates driven by an influenza A(H3N2) strain in HHS Region 10, which includes the Pacific Northwest states: Alaska, Idaho, Oregon, and Washington.
The CDC emphasizes that getting vaccinated is a crucial preventive measure, even late in the current flu season, as it significantly reduces the risk of severe illness, hospitalization, and death.
The CDC strongly advises those who have not yet been vaccinated to do so as soon as possible. Various flu vaccines are still available at local clinics throughout the USA.
Approximately 134 million doses of influenza vaccine have been distributed in the United States this season.
For the most up-to-date information, including interactive maps and detailed regional data, please visit the CDC's FluView portal at cdc.gov/fluview.
As the February 2026 vaccination plans are being formed, the UK Health Security Agency (UKHSA) has issued an important reminder for travelers heading to Cape Verde to take extra precautions against gastrointestinal infections when visiting the West African archipelago.
As of February 5, 2026, recent UKHSA data indicate a significant cluster of Shigella sonnei cases, a bacterial cause of severe dysentery-like illness, along with multiple clusters of non-typhoidal Salmonella infections strongly linked to recent travel to Cape Verde.
Since October 1, 2025, the UKHSA has confirmed 158 cases of Shigella sonnei in travelers returning to England, Scotland, and Wales. Of the 118 cases where international travel was reported, 112 (94.9%).
Many travelers to Cape Verde have visited the Santa Maria area on Sal Island and the Boa Vista area, popular resorts known for their beaches.
The UKHSA says this strain of Shigella sonnei shows no genomic resistance to the common antimicrobials typically used to treat traveler's diarrhea, which could facilitate treatment if an infection occurs.
Additionally, 32 cases were linked to travel to Cape Verde during the same period.
Investigations into the outbreaks are ongoing and involve international health partners. Cases have also been reported in other European countries, particularly from September to November 2025.
Shigella and Salmonella are primarily transmitted through contaminated food and water, poor hygiene practices, and person-to-person contact. The risk of infection is especially heightened in resort settings.
Symptoms typically appear within a few days, and while most people recover within a week with supportive care (with hydration being crucial), severe cases can lead to dehydration, hospitalization, or complications. This is particularly concerning for vulnerable groups such as young children, the elderly, and individuals with weakened immune systems.
The UKHSA emphasizes that while Cape Verde is a popular travel destination, increased vigilance can help prevent illness and ensure a safe holiday. Travelers who experience symptoms upon returning should seek medical advice and inform their doctor about their travel history.
From a prevention standpoint, no Shigella vaccine has received regulatory approval from any health authority as of February 2026.
However, the Shigella4V (S4V or S4V2) vaccine candidate received U.S. FDA Fast Track designation in 2024. Phase 2 studies (including in infants) are underway, but no Phase 3 completion or approval has been achieved yet.
In a recent update from the U.S. Centers for Disease Control and Prevention (CDC), details were released about a gastrointestinal illness (GI) outbreak aboard the Regent Seven Seas cruise ship, Seven Seas Mariner.
The CDC reported this incident on February 2, 2026, which affected a small percentage of passengers and crew, underscoring the ongoing challenges of maintaining health standards on luxury cruises in the travel environment.
According to the CDC's Vessel Sanitation Program (VSP), the final case counts showed that 21 of 631 passengers (3.3%) reported illness, and 6 of 458 crew members (1.3%).
The predominant symptom among those affected was diarrhea, and the specific causative agent remained unknown despite testing.
In response to the outbreak, Regent Seven Seas and the ship's crew implemented several measures as outlined in the CDC report. These included enhancing cleaning and disinfection procedures in accordance with their established outbreak prevention and response plan. Stool specimens were collected from affected individuals for laboratory testing, and ill passengers and crew members were isolated to prevent further spread.
The cruise line consulted directly with VSP officials to enhance sanitation protocols and improve case reporting.
Regent Seven Seas has consistently received high scores from the VSP, often exceeding 95 out of 100 in routine inspections. This strong track record may have helped in the swift containment of the recent outbreak.
The CDC says GI outbreaks are common in close-quarters environments, such as cruise ships, and are often linked to norovirus or other pathogens. However, the relatively low infection rate in this case indicates that the situation was effectively managed.
In 2025, there were 23 reported outbreaks, of which 17 were attributed to norovirus. This virus is the most common cause of confirmed GI outbreaks on cruise ships because it spreads easily in tight spaces.
For those planning cruise ship voyages in 2026, Vax-Before-Travel.com reports that no vaccine is currently available for norovirus.

Measles is an ongoing risk around the world, and children are at serious risk for the highly transmissible disease in most countries. Since 2023, measles incidence has been rising in many countries worldwide, including in Europe.
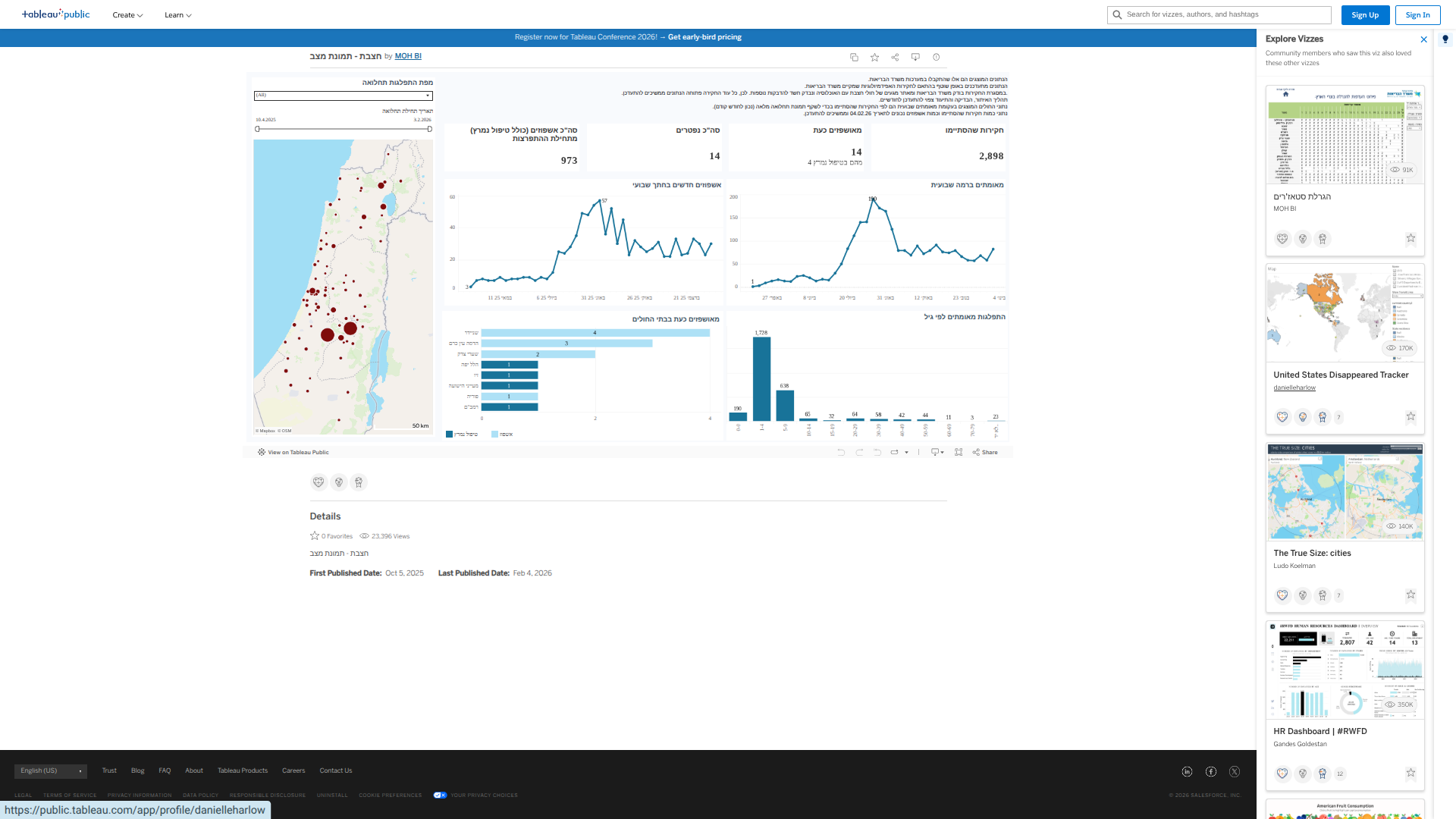
The Israeli Ministry of Health recently reported the 14th pediatric fatality due to measles since an outbreak began in mid-April 2025, partially from imported cases and others of unknown origin.
The Ministry's announcement on February 2, 2026, confirmed that the affected individual was a one-year-old who had not been vaccinated. The baby was brought to Hadassah Mount Scopus Hospital in Jerusalem in critical condition.
Similar to previous cases, most of the deceased were healthy infants without any underlying conditions and had also not received vaccinations.
The Ministry emphasizes that measles is a preventable disease and advises that all children should receive their first vaccine dose at the age of one. In areas affected by the outbreak in Israel, the Health Ministry recommends administering the second dose of the measles vaccine at 18 months.
Additionally, an extra vaccine dose is recommended for infants aged 6 to 11 months in outbreak areas and for those traveling to outbreak areas.
As of February 5, 2026, the areas in Israel that have been classified as experiencing a measles outbreak are Jerusalem, Beit Shemesh, Bnei Brak, Harish, Modi'in Illit, Nof HaGalil, Kiryat Gat, Ashdod, Safed, Netivot, Haifa, Tiberias, the Mateh Binyamin Regional Council, and the settlement of Tekoa.
To alert international travelers to their health risk, the U.S. CDC has maintained a Level 1, Travel Health Notice, that identifies measles outbreaks in numerious countries. The CDC advises all travelers to speak with a local travel vaccine expert about measles immunization options and other vaccine-preventable diseases.
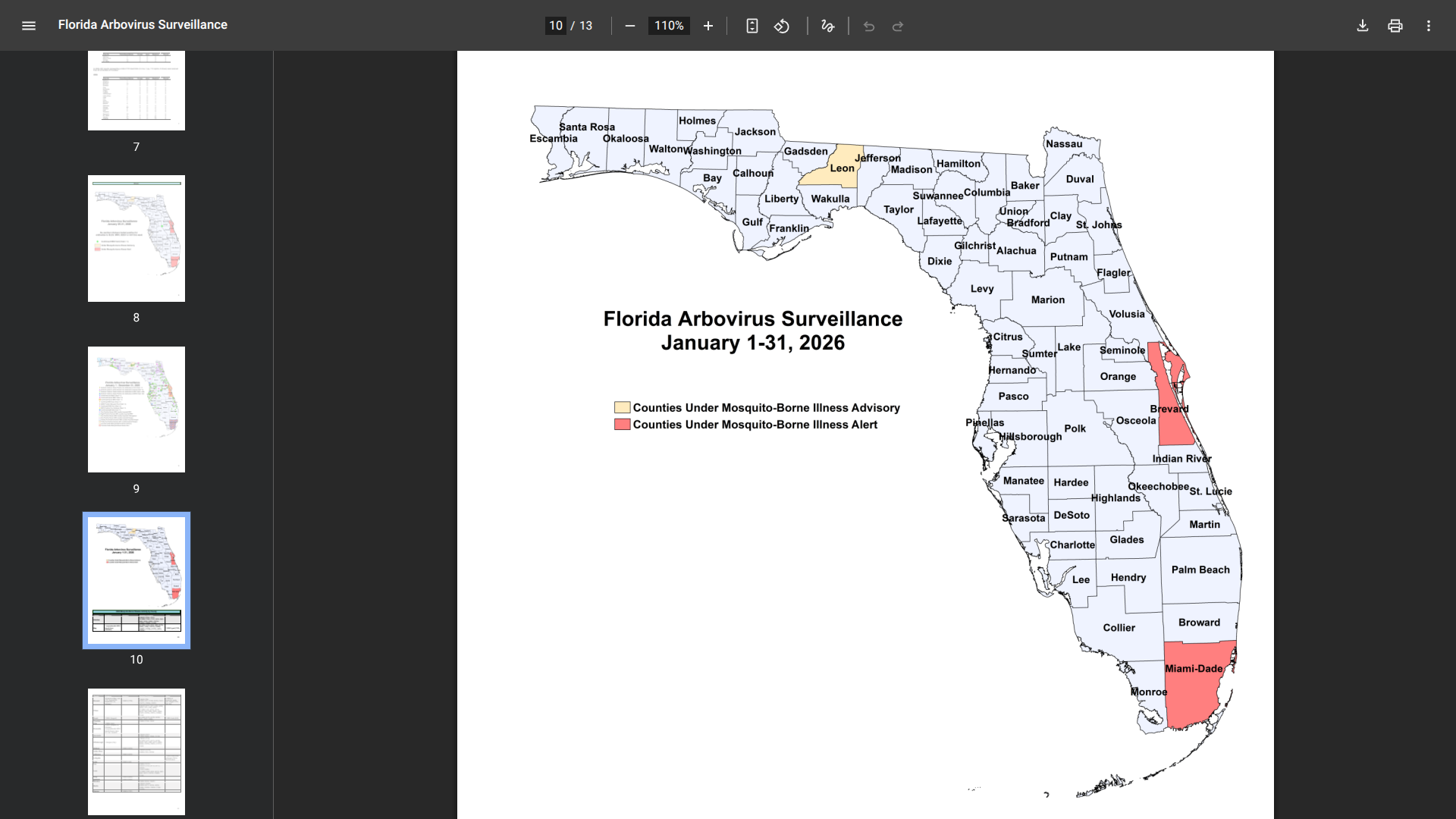
The Florida Department of Health (DOH) today announced updated surveillance data on chikungunya fever cases in the state.
As of February 4, 2026, the data emphasize the ongoing risks associated with travel to chikungunya-endemic areas, particularly Cuba, as well as the rare but noteworthy occurrence of local transmission in Florida.
As of the latest reporting period (#4) in 2026, nine cases have been confirmed among individuals with travel history to chikungunya-endemic areas within two weeks before onset, all linked to travel to Cuba.
And one case of locally acquired chikungunya fever was reported in Miami-Dade County, with symptom onset in December 2025.
In 2025, a total of 350 chikungunya cases were reported among individuals who had recently traveled to chikungunya-endemic areas. These cases were distributed across multiple Florida counties, with Miami-Dade County reporting the highest number at 229.
Chikungunya fever is a viral illness primarily transmitted by Aedes mosquitoes. Symptoms include high fever, severe joint pain, muscle pain, headache, nausea, fatigue, and rash. Most individuals recover completely; however, joint pain can be debilitating and may persist for months in some cases. There is no specific antiviral treatment, but supportive care can help relieve symptoms.
The DOH and the U.S. CDC advise travelers to endemic areas to consult local healthcare clinics about preventive strategies, including vaccination options. In Florida and most states, U.S. FDA-approved chikungunya vaccines are available in 2026.