Search API
The Hawai‘i Department of Health (DOH) recently confirmed its ninth travel-related case of dengue in 2025.
As of the end of June 2025, the DOH reported eight dengue cases on Oʻahu and one case on Maui.
Several countries in the Pacific Region are reporting an increase in mosquito-transmitted dengue virus cases, including Fiji, French Polynesia, Tonga, and the Republic of the Philippines.
According to the World Health Organization, dengue is a grade 3 emergency, with an estimated 4 billion people at risk globally.
The U.S. Centers for Disease Control and Prevention (CDC) reissued a Global Travel Health Notice on June 18, 2025, regarding dengue outbreaks in the Asia/Pacific region, including India, Singapore, Thailand, the Philippines, Malaysia, and Myanmar.
CDC reported on July 3, 2025, that 2,248 travel-related Dengue cases and two local cases in Miami, Florida, were reported in 41 jurisdictions this year.
While the CDC currently authorizes the first-generation dengue vaccine for use in Puerto Rico, where dengue has become endemic, numerous countries enable access to a second-generation vaccine in 2025. Additionally, innovative dengue vaccine candidates are making progress in late-stage clinical trials.

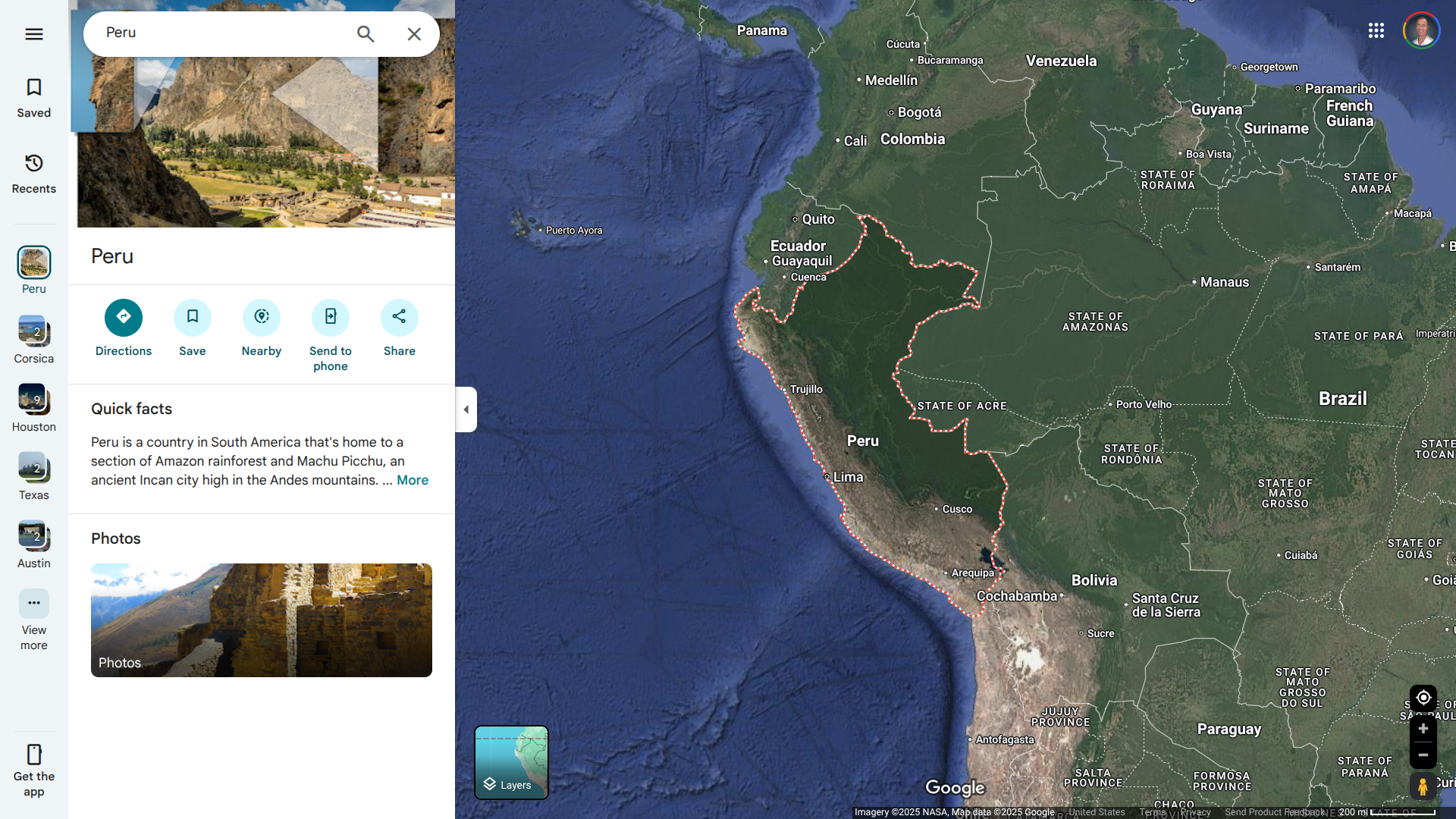
The Republic of Peru frequently declares regional states of emergency, particularly near border areas.
To highlight these high-risk zones, the Canadian government updated its travel advisory for Peru on July 4, 2025, informing travelers to exercise a high degree of caution in Peru due to civial unrest that may occur across the country.
These areas include:
Huallaga and Tocache provinces in the department of San Martín,
the Upper Huallaga and Ene river valleys in the departments of Huánuco and San Martín,
Padre Abad province in the department of Ucayali,
Huacaybamba, Humalíes, Leoncio Prado, and Marañón provinces in the department of Huánuco,
Concepción and Satipo provinces in the department of Junín,
Tayacaja province in the department of Huancavelica,
the districts of Abancay, Andahuaylas, and Chincheros in the department of Apurímac,
Huanta and La Mar provinces, in the department of Ayacucho,
Valley of Apurimac, Ene, and Mantaro rivers (VRAEM).
Previously, the U.S. Department of State reissued its Level 2: Exercise Increased Caution Travel Advisory for this South American country on May 16, 2025.
From a health risk perspective, the U.S. CDC includes Peru in Travel Health Advisories for measles, Oropouche, and yellow fever.
The CDC recommends that visitors to Peru consider several routine and travel vaccines, such as typhoid and chikungunya, before traveling abroad in 2025.
These advisories are essential, as over 3 million international visitors traveled to Peru in 2024, representing a significant increase in activity compared to 2023. Many travelers visited Machu Picchu, located high up in the Andes Mountains.
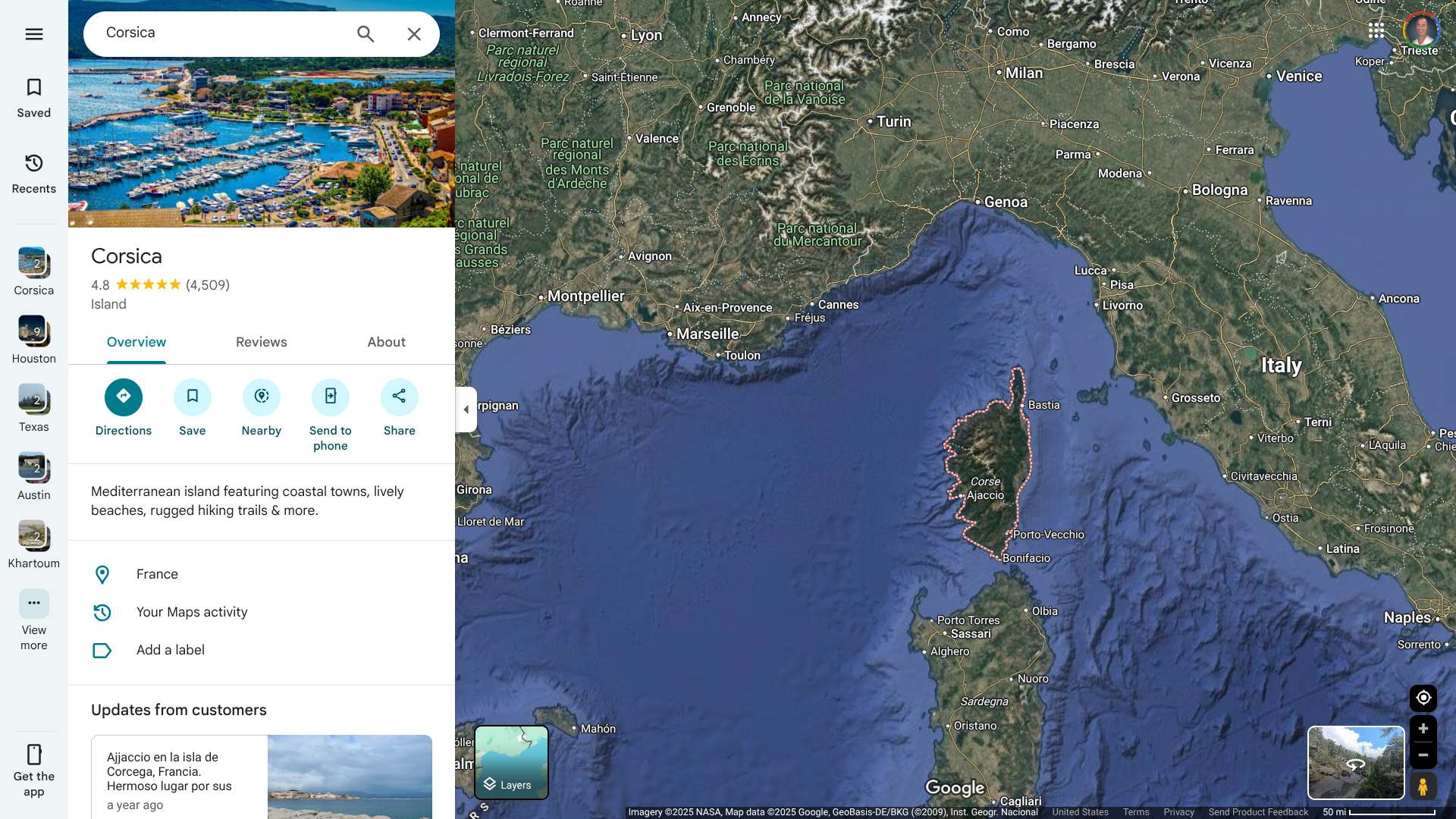
The French Health Ministry has been reporting a significant number of locally acquired chikungunya cases in its Department in 2025. ARS Corsica states that the presence of the virus-carrying tiger mosquito is now well established in southern Corsica and throughout Europe.
As of July 2, 2025, a third locally acquired case of chikungunya has been detected in southern Corsica, in Porticcio (municipality of Grosseto-Prugna).
In late June 2025, ARS Corsica reported two cases of chikungunya from the same family, residing in Grossetto-Prugna.
On France's mainland, the Occitanie Regional Health Agency detected a locally transmitted case of Chikungunya in the Hérault Department on June 16, 2025. Another case was reported in La Crau (Var) on June 11, 2025.
Additionally, the Departments of Reunion and Mayotte in the southern India Ocean have been confronting chikungunya outbreaks over the last few months.
From a health protection perspective, chikungunya vaccines are now commercially available in France and the United States.
The U.S. CDC recommends that international travelers visiting areas with chikungunya outbreaks speak with a travel vaccine expert about immunization options.
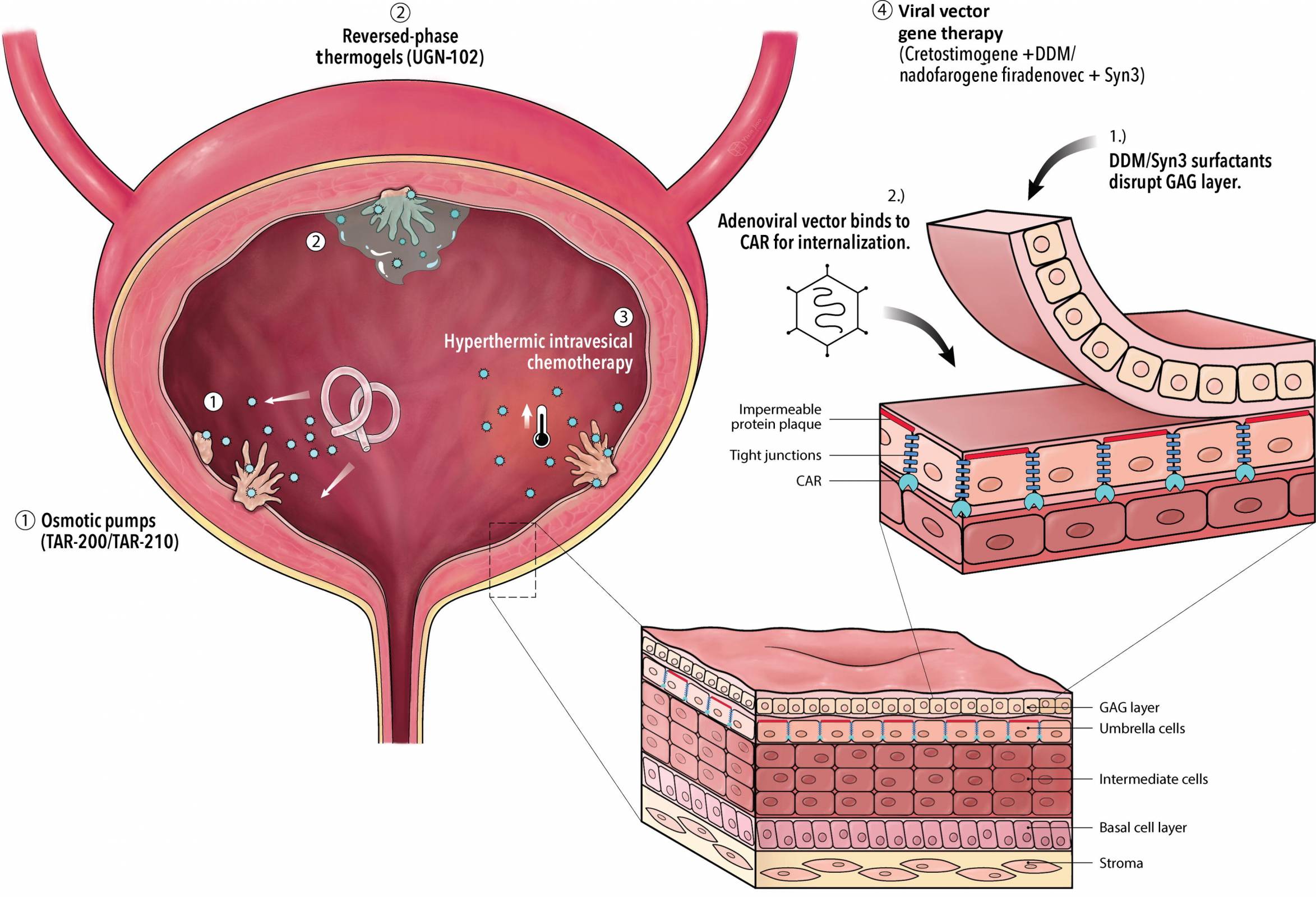
An innovative bladder cancer intravesical therapy today gained its initial approval outside the United States. Bladder cancer is a challenging malignancy, and for many years, researchers have searched for next-generation treatment options.
The UK's Medicines and Healthcare products Regulatory Agency (MHRA) has approved nogapendekin alfa inbakicept (Anktiva®) for adults with BCG-unresponsive non-muscle-invasive bladder cancer, where the disease remains confined to the inner lining of the bladder and may include tumors.
Nogapendekin alfa inbakicept (Anktiva) mixed with BCG is administered via a liquid that is diluted and then delivered into the bladder through a catheter inserted into the urethra.
The BCG (Bacillus Calmette-Guérin) vaccine, which has been deployed for approximately 100 years to reduce tuberculosis, has become a standard immunotherapy for early-stage bladder cancer. It is delivered directly into the bladder to stimulate an immune response.
Anktiva's mechanism of action involves the direct, specific stimulation of CD8+ T cells and Natural Killer cells through beta-gamma T-cell receptor binding, thereby generating memory T cells while avoiding stimulation of T-regulatory cells.
As of July 4, 2025, this medicine was approved through the International Recognition Procedure. The approval was granted to Serum Life Science Europe GmbH.
On May 27, 2025, ImmunityBio announced a collaboration to introduce the Cancer BioShield platform, along with Anktiva, to Saudi Arabia and the broader Middle East.
In the United States, ImmunityBio, Inc.'s BioShield platform, powered by Anktiva, was approved by the U.S. FDA for similar indications in April 2024. It is now commercially available at over 60 cancer centers in the U.S.
On July 1, 2025, ScienceDirect published a systematic review highlighting an array of novel intravesical therapies that demonstrate efficacy in bladder cancer patients.
A new study, published this week in JAMA Network Open and conducted by researchers from the University of Kentucky, analyzed data from the Cancer Statistics Incidence Analytic Database.
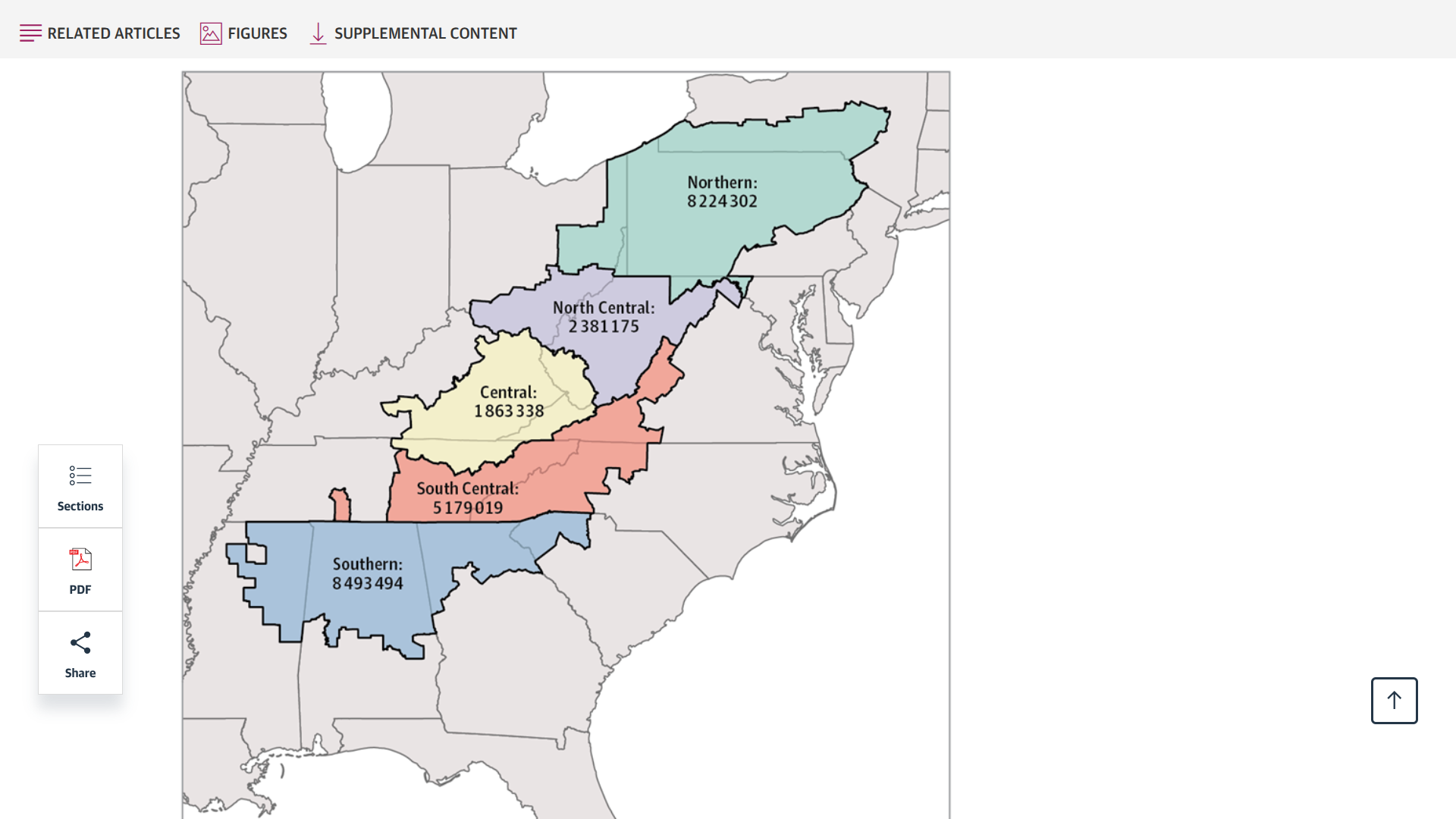
Released on June 30, 2025, this study's findings underscore the significant burden of cancer linked to human papillomavirus (HPV) among residents of Appalachia, who have a 16% higher rate of HPV-related cancers.
Furthermore, regional-specific disparities were seen for HPV-associated male and female oropharyngeal cancers, female anal cancer, vulvar cancer, cervical cancer, and penile cancer.
Incidence was highest among individuals living in the North Central and Central regions.
The North Central subregion had the highest incidence rates of male oropharyngeal cancer.
In contrast, the Central and North Central subregions had significantly higher rates of vulvar, cervical, and penile cancers than the other subregions.
These researchers wrote, 'This cross-sectional study of HPV-associated cancer incidence found disproportionately high HPV-associated cancer rates among Appalachian residents compared with non-Appalachian residents.'
'These findings highlight the need for targeted efforts to improve HPV vaccine uptake and encourage adherence to evidence-based screening guidelines for HPV-associated cancers in Appalachia.'
Currently, the U.S. CDC recommends HPV vaccination for most adolescents in a two or three-dose regimen. And recommends vaccination for everyone through age 26 if not adequately vaccinated at a younger age.
As of July 3, 2025, Merck's Gardasil 9 vaccine is readily available at health clinics and pharmacies throughout the Appalachian Mountain range and the United States.
Following a significant outbreak in 2024, the first confirmed case of West Nile virus illness was reported on June 24, 2025, in a resident of Brazos County, Texas.
This Texas Department of State Health Services (DSHS) announcement raises concerns for all Texans as virus-carrying mosquitoes remain active into December.
There were 455 cases of West Nile disease in Texas in 2024 and 56 related fatalities.
Over the last five years, Texas has had 929 West Nile cases and 122 deaths.
“Texans should be aware that mosquitoes transmit disease, and some of these illnesses, like West Nile and dengue, can be severe,” said DSHS Commissioner Jennifer A. Shuford, MD, MPH. “But taking steps to prevent mosquito bites and eliminating mosquito breeding areas around homes are proactive measures that can reduce the risk of mosquito-borne illness.”
Infected mosquitoes transmit West Nile virus after biting. Although 80% of people exposed to the virus do not develop symptoms, the remaining 20% will experience symptoms such as fever, nausea, headache, fatigue, and muscle and joint pain.
Less than one percent of those exposed will suffer from West Nile neuroinvasive disease, which affects the nervous system and can cause disorientation, neck stiffness, tremors, paralysis, convulsions, and even death.
DSHS urges anyone experiencing West Nile symptoms to contact their health care provider and mention any exposure to mosquitoes.
As of July 3, 2025, no vaccine for West Nile virus exists.

Florida health officials recently reported additional cases of mosquito-transmitted and travel-related chikungunya, dengue, and malaria in 2025.
After a slow start, these serious diseases have been concentrated in southeast Florida, in the great Miami area, which welcomes millions of international visitors each year.
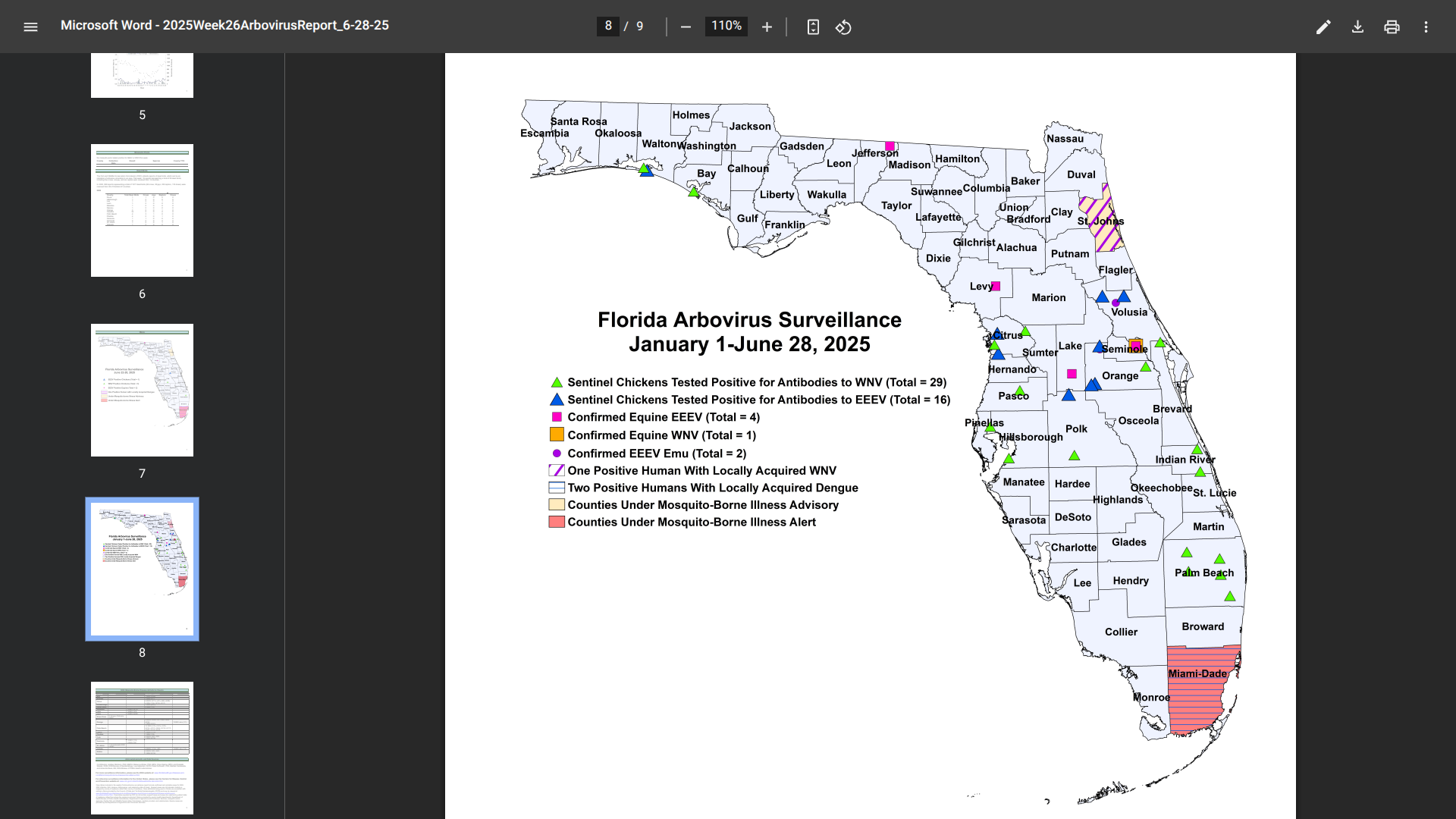
Currently, Miami-Dade County remains under a mosquito-borne illness alert.
As of June 28, 2025, Florida Health's Weekly Arbovirus Report confirmed a second, indigenous case of dengue in Miami-Dade County.
The most recent case has been identified as serotype DENV-4, while the early case was DENV-3.
In 2024, a total of 91 cases of locally acquired dengue have been reported across ten Florida counties.
Additionally, there have been four cases of chikungunya and 20 cases of malaria related to international travelers this year.
While there are no travel advisories issued for Florida's southeast coast, Canada and the UK both advise speaking with a travel vaccine expert before visiting at-risk areas in 2025.