Florida Prepares for the 2025-2026 RSV Season
While most people are aware that the Respiratory syncytial virus (RSV) is a common respiratory virus, many are unaware that outbreaks are typically first detected in Florida each year.
And these outbreaks persist longer than in the rest of the nation.
Based on data from the new Florida Department of Health (FDH) RSV Review Activity Summary, it's time to take preventive actions in Florida.
FDH states that identifying unique seasonal and geographic trends in RSV activity in Florida has significant implications for prescribing patterns and the initiation of prophylaxis for children and seniors at high risk for complications from RSV infection.
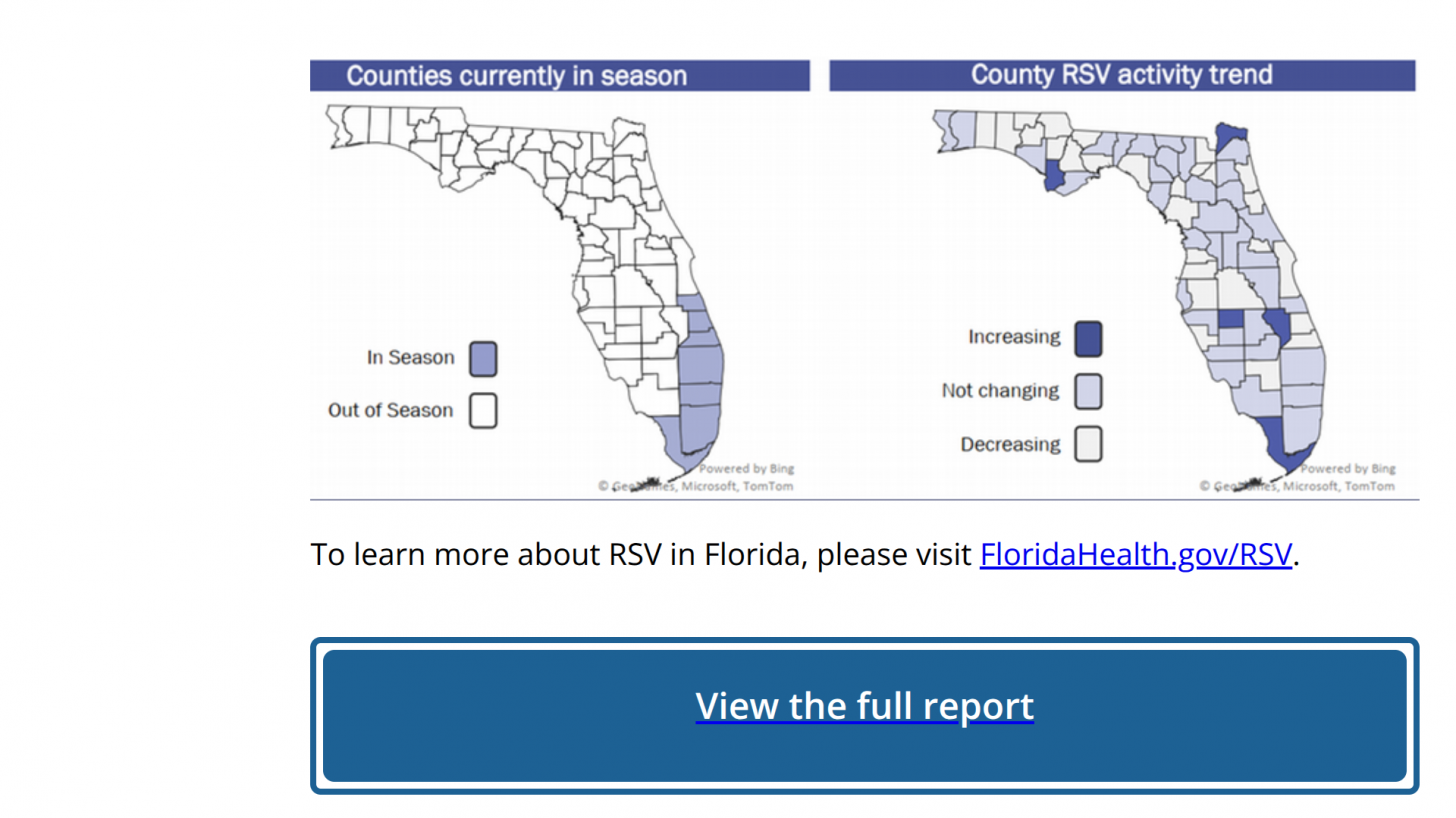
As of June 28, 2025, RSV detections have been confirmed along Florida's southeast coast, which includes Miami and West Palm Beach, as well as on the west coast, including Ft Myers.
Since 2023, vaccines and a monoclonal antibody passive immunization have been approved for the prevention of RSV-related illness.
In 2025, the American Academy of Pediatrics currently recommends that pre-approval for prophylactic treatment be made based on state surveillance data.
As of last month's FDA/CDC approval, a second long-acting, monoclonal antibody is now available in Florida and throughout the United States.
On June 28, 2025, Merck announced that ENFLONSIA™ (clesrovimab-cfor) was approved for the prevention of RSV lower respiratory tract disease in neonates (newborns) and infants born during or entering their first RSV season. ENFLONSIA is designed to provide direct, rapid, and durable protection for up to 5 months, a typical RSV season, with a single 105 mg dose regardless of weight.
"Ahead of the 2025-2026 RSV season, we are proud to offer ENFLONSIA as a new preventive option designed to protect healthy and at-risk infants from RSV disease across a spectrum of severity, including worsening disease requiring hospitalization," commented Dr. Richard M. Haupt, vice president, head of global medical & scientific affairs, vaccines and infectious diseases, Merck Research Laboratories, in a press release.
Merck stated that, beginning with shipments in July, it is committed to ensuring the availability of ENFLONSIA in the U.S. before the start of the upcoming RSV season, aiming to help reduce the significant burden of this widespread seasonal infection on families and healthcare systems.
Our Trust Standards: Medical Advisory Committee
























