Search API
The World Health Organization (WHO) announced today that health authorities in Ethiopia are increasing their response and conducting further investigations following reports of suspected cases of viral hemorrhagic fever in the South Ethiopia Region.
As of November 13, 2025, the WHO has reported eight suspected cases in this region where about 7.5 million people live.
The South Ethiopia Regional State borders Kenya and South Sudan.
Laboratory testing is currently underway to determine the exact cause of these cases. Viral hemorrhagic fever is caused by several distinct families of viruses, including Marburg, Ebola, Crimean-Congo hemorrhagic fever, and Lassa fever.
Currently, only the Zaire Ebola virus has approved vaccines and antibody therapies.
In support of this investigation, the WHO has deployed an initial team of responders and delivered medical supplies to assist in the ongoing efforts to determine the cause of infection and halt further transmission.
Previously, the U.S. CDC included Ethiopia in its Travel Health Notices for malaria, measles, and polio issued in 2025.
Malaria infections in sub-Saharan Africa are increasingly showing resistance to artemisinin-based therapies, posing a significant threat to the effectiveness of antimalarials against the mosquito-transmitted parasite.
However, a novel non-artemisinin antimalarial from Novartis has demonstrated that it is as effective as standard treatment.
On November 12, 2025, Novartis announced positive results from KALUMA, a Phase III study for the new malaria treatment KLU156 (ganaplacide/lumefantrine, or GanLum).
This drug was developed with Medicines for Malaria Venture and met the study's primary non-inferiority endpoint relative to the current standard of care. The treatment achieved a 97.4% PCR-corrected cure rate using an estimand framework, compared to 94% with standard of care.
This data equates to cure rates of 99.2% and 96.7% respectively, based on conventional per-protocol analysis.
"GanLum could represent the biggest advance in malaria treatment for decades, with high efficacy against multiple forms of the parasite as well as the ability to kill mutant strains that are showing signs of resistance to current medicines," said Dr Abdoulaye Djimdé, Professor of Parasitology and Mycology at the University of Science, Techniques and Technologies of Bamako, Mali, in a press release.
"Drug resistance is a growing threat to Africa, so new treatment options can't come a moment too soon."
GanLum is a combination of two compounds that attack the malaria parasite on multiple fronts: ganaplacide, a novel compound with an entirely new mechanism of action, and a new once-daily formulation of the existing antimalarial lumefantrine, a longer-acting treatment.
Ganaplacide is believed to work by disrupting the parasite's internal protein transport systems, which are essential to its survival within red blood cells.3 It belongs to a class of compounds called imidazolopiperazines, first identified as potential antimalarials after a groundbreaking screen of 2.3 million molecules to find drug candidates at Novartis labs in San Diego, California.
Novartis plans to seek regulatory approvals from health authorities for GanLum as soon as possible.
If approved, the drug could be used to treat international travelers returning to the United States after being infected.
For example, in Florida, 43 travel-related malaria cases have been confirmed in 2025, 15 related to travel to Nigeria.
From a malaria prevention option, about 24 countries are now offering malaria vaccinations, but not the USA.
Germany's public health agency announced yesterday that Wild poliovirus type 1 (WPV1) had been recently detected in a sewage sample within this European country.
According to the Federal Ministry, the WPV1 virus likely entered Germany through an individual infected in either Pakistan or Afghanistan, the only two countries where wild polioviruses continue to spread. This type of poliovirus can cause poliomyelitis in people who are not vaccinated or are only partially vaccinated.
The Robert Koch Institute (RKI) wrote on November 12, 2025, that the risk to the general population from polioviruses is considered very low due to high vaccination rates and isolated detection in wastewater. No clinical cases of poliomyelitis have been reported to the RKI to date.
However, the occurrence of a clinical case in unvaccinated individuals cannot be ruled out.
Complete polio vaccination with the inactivated polio vaccine (IPV) used in Germany provides reliable protection against the disease, but only limited protection against infection and transmission.
In September 2025, Germany previously published answers to frequently asked questions about poliomyelitis with a focus on wastewater analysis.
A detailed article on this topic will be published in Epidemiological Bulletin 46/2025 on November 13, 2025.
Currently, Germany is mentioned in the U.S. CDC's Global Polio Travel Health Notice. The CDC also recommends that international travelers be vaccinated with the IPV before traveling abroad in 2025.
Infection with the mpox virus (MPXV) confers stronger immunity against future infection than vaccine-conferred protection, which wanes over time and requires boosting, researchers wrote in The Lancet Infectious Diseases on November 7, 2025.
These findings suggest that MPXV infection confers long-term protection against reinfection, whereas vaccine-induced immunity can wane over time and requires boosting.
These researchers wrote that further studies are needed to determine whether booster doses can enhance the durability of immunological memory in previously vaccinated individuals.
Should booster vaccination prove beneficial, targeted revaccination campaigns will be necessary to maintain population-level protection.
In a related commentary, researchers from the Icahn School of Medicine at Mount Sinai said that the study's findings indicate that the success of next-generation vaccines against orthopoxviruses, such as mpox, will depend on antigen selection that focuses immune responses on proteins associated with long-lasting protection and cross-reactivity against multiple orthopoxviruses.
Currently, the U.S. FDA-approved JYNNEOS (MVA-BN) mpox/smallpox vaccine is offered at clinics and pharmacies in the United States.
Funding for this study was from the Research Foundation–Flanders, Department of Economy, Science and Innovation Flanders, and the Netherlands Organization for Health Research and Development.

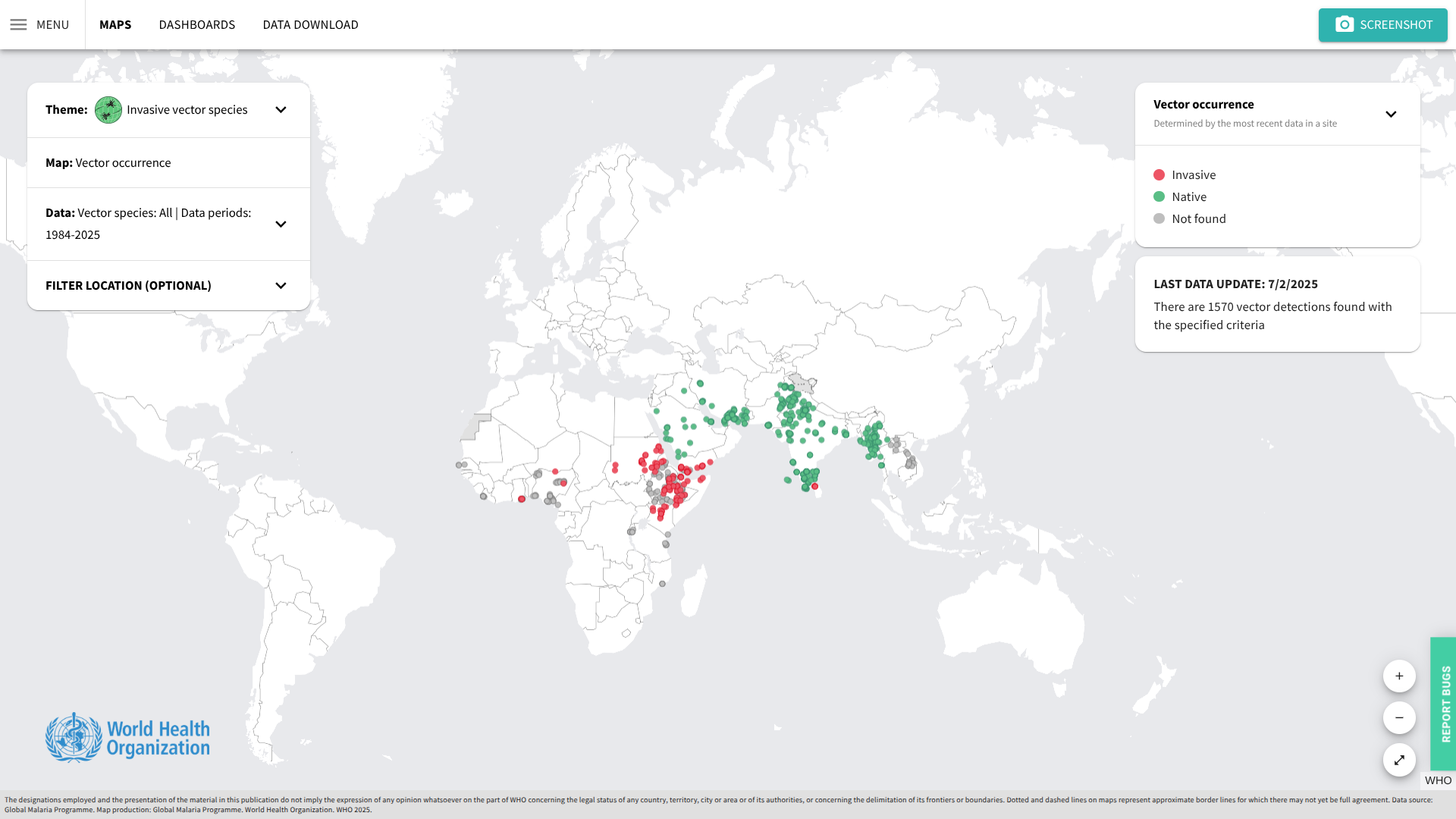
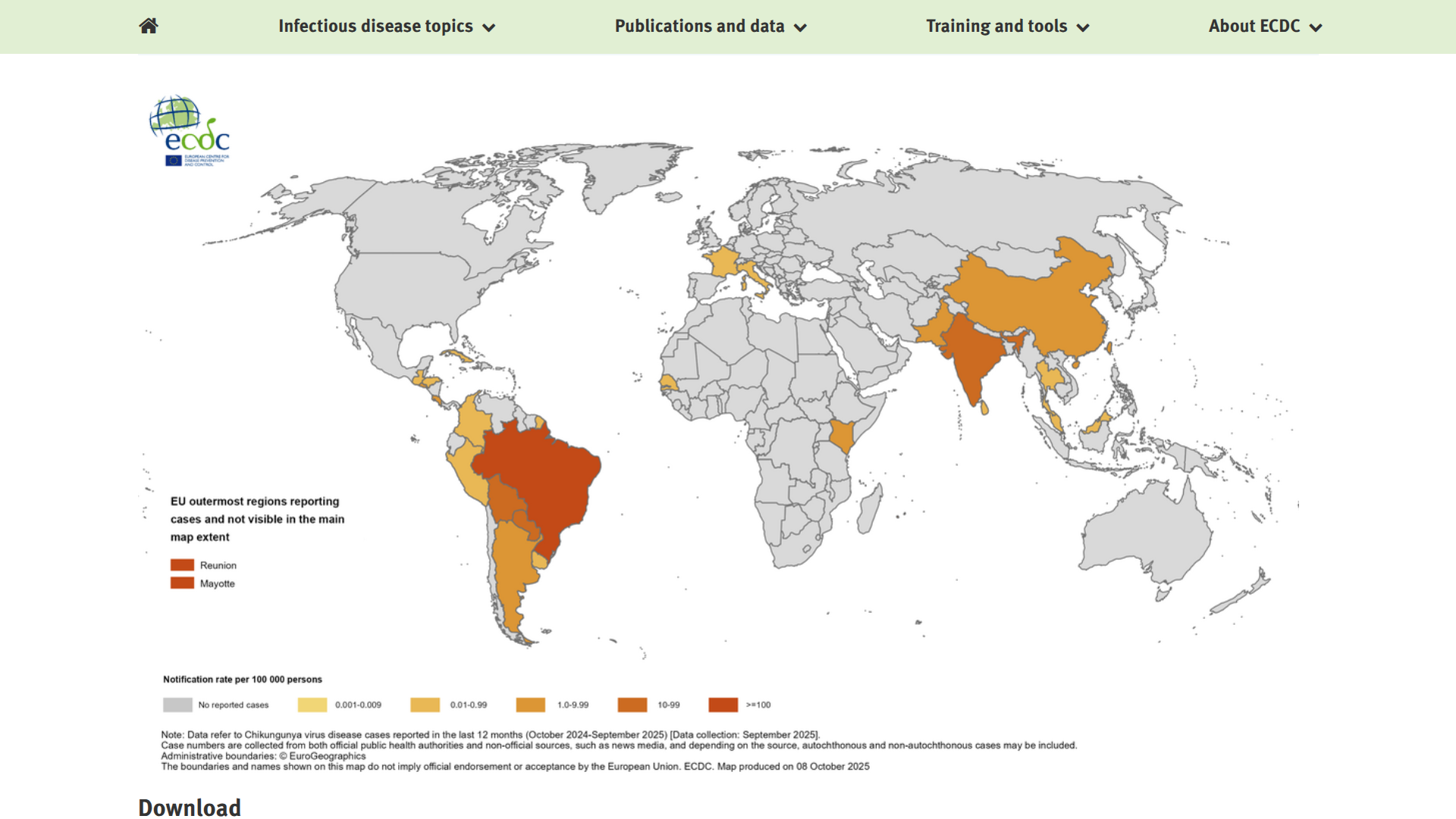
Chikungunya, a viral infection caused by an alphavirus that is spread to people through the bite of an infected female Aedes mosquito, significantly impacted the Americas and the European region in 2025.
According to the World Health Organization (WHO) latest report, a total of 445,271 suspected and confirmed Chikungunya cases and 155 related fatalities worldwide. The distribution of cases across regions has been uneven, with some countries reporting a resurgence in numbers during 2025.
The French Overseas Departments in the Indian Ocean, such as La Réunion, are Europe's unfortunate leader in Chikungunya outbreaks in 2025.
The WHO wrote that the potential for further geographical spread is highlighted by the fact that 27 countries and territories have established competent vector populations (Aedes aegypti and Aedes albopictus mosquitoes) but have not yet documented local Chikungunya transmission.
In the United States, as of November 11, 2025, 88 travel-related Chikungunya cases have been confirmed by the U.S. CDC.
The state of Florida, which is geographically located near endemic areas such as Cuba, has reported 42 travel-related Chikungunya cases confirmed this year in Broward, Collier, Lake, Miami-Dade, Palm Beach, and Seminole Counties.
And recently, the New York State Department of Health confirmed a locally acquired case of Chikungunya in Nassau County.
The CDC does recommend Chikungunya vaccination for specific international travelers in 2025.
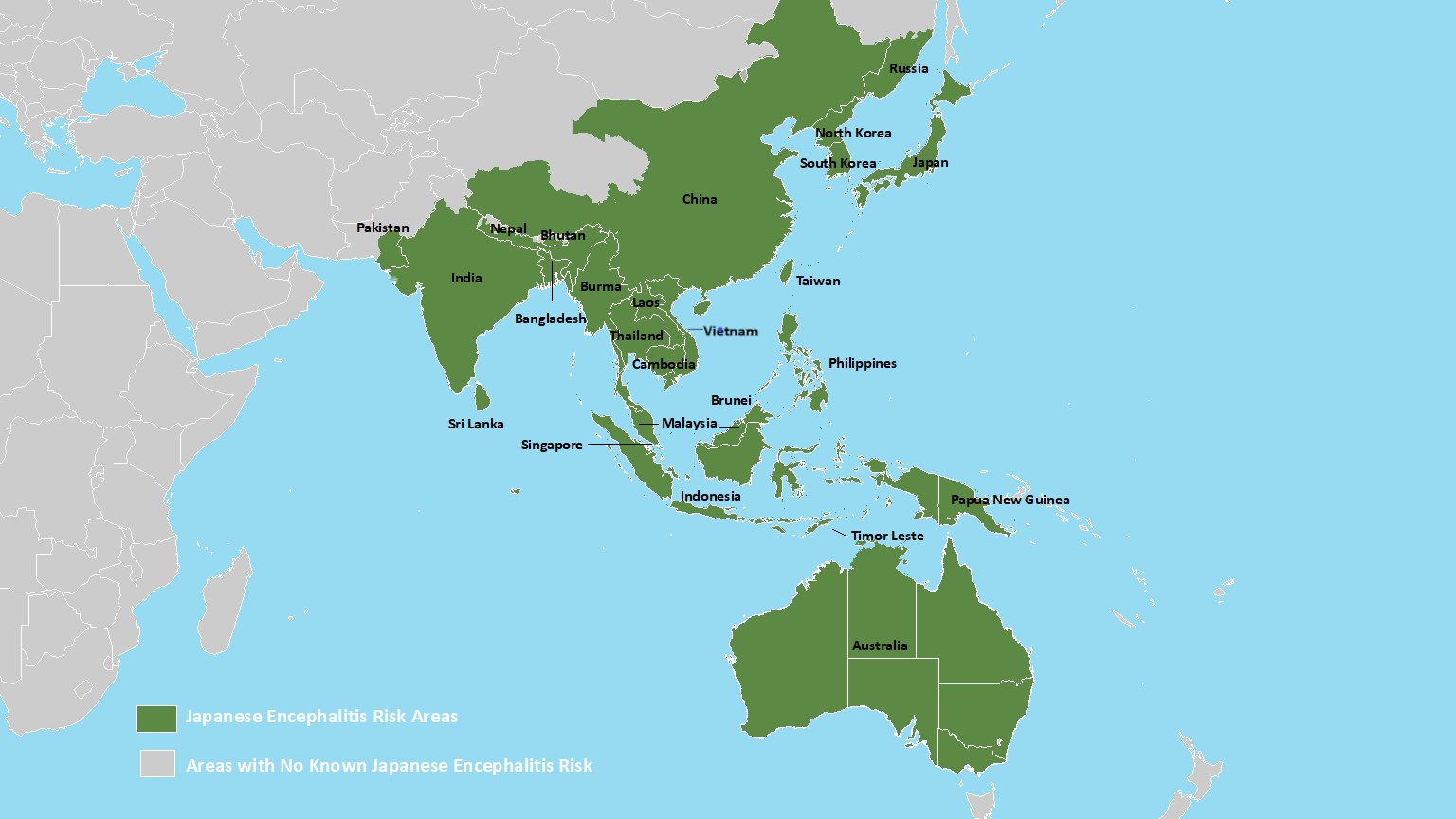
Japanese encephalitis virus (JEV) is the leading cause of epidemic encephalitis worldwide and is prevalent across Asia and the Pacific.
A recent study published in the journal Nature highlights significant public health concerns stemming from changes in the dominant genotype, the emergence of epidemics in new regions, and the re-emergence of previously dormant genotypes.
The re-emergence of specific genotypes in Indonesia after 37 years, coupled with JEV-related fatalities in Australia, Nepal, and Taiwan, highlights the need for critical control measures.
Nepal's Department of Health Services has confirmed 164 cases of Japanese encephalitis in 2025, compared to 86 last year.
In 2024, 23 people died in Nepal infected with the JEV.
Similarly, the resurgence of genotype in China after 57 years and its circulation in Korea underscore the need for continuous surveillance and proactive vaccinations.
According to the U.S. CDC, a Japanese encephalitis vaccine (IXIARO) is available in the United States, approved for use in children aged 2 months and older and adults.
The CDC says this approved vaccine should be considered for some travelers before visiting high-risk areas in 2025.
In the United States, IXIARO is commercailly offered at travel clinics and pharmacies.