Oropouche and Zika Viruses Cause Microcephaly in Infants
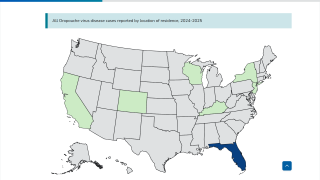
The U.S. Centers for Disease Control and Prevention (CDC) recently confirmed that the re-emergence of the Oropouche Virus in the Region of the Americas is creating severe health issues, and some of these are similar to the Zika virus.
Zika virus was first recognized in Uganda in 1947, and outbreaks continue in 2024.
The CDC published two Early Release Emerging Infectious Diseases (Volume 30, Number 11—November 2024; Volume 30, Number 12—December 2024) regarding the ongoing Oropouche virus outbreak in the Americas.
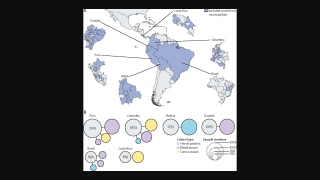
Oropouche has been documented in both urban and rural areas in Bolivia, Brazil, Colombia, Cuba, Dominican Republic, Guyana, and Peru. Infection rates are high, up to 30% of the population.
An analysis of about 5,000 confirmed cases identified in Brazil found that most occurred in persons 20–49.
The incubation period for Oropouche virus disease ranged from 3 to 10 days, and 60% of infected persons experienced symptoms. Symptoms are similar to those of other vectorborne diseases, Zika, and include acute onset of fever and severe headache, often with chills, myalgia, arthralgia, and fatigue.
Up to 70% of people can report a relapse of symptoms after the initial illness, typically within a few days to weeks. Secondary episodes are clinically similar to the primary episode.
Of two recent deaths associated with the Oropouche virus among previously healthy young adult women, at least one patient had signs of hemorrhage (nasal, gingival, and vaginal bleeding and petechiae) starting four days after initial symptom onset.
Signs and symptoms of neurologic disease can include occipital pain, dizziness, limb weakness, paresthesia, confusion, lethargy, photophobia, nausea, vomiting, nuchal rigidity, nystagmus, and paralysis.
In June 2024, vertical transmission of the Oropouche virus was identified when RNA was detected in a stillborn infant born to a pregnant woman who had symptoms of Oropouche virus disease at 30 weeks gestation.
After this identification, a retrospective investigation identified four infants with microcephaly in whom Oropouche virus IgM was detected in serum samples or cerebrospinal fluid (CSF) samples collected shortly after birth.
In August 2024, an additional infant with microcephaly associated with Oropouche virus infection was reported. The infant, born in June 2024, tested positive for Orepouche virus IgM in serum and CSF on the second day of life.
The infant later died at 47 days of life, and multiple tissues tested positive for Oropouche viral RNA.
Additionally, the December study describes the shedding of Oropouche virus (OROV) RNA in a symptomatic traveler's whole blood, serum, and urine. Virus replication was detected in semen 16 days after infection, which the authors say suggests a risk of sexual transmission.
'Our findings raise concerns over the potential for person-to-person transmission of OROV via sexual encounters. Pending further evidence, we recommend the use of barrier protection when engaging in sexual intercourse if OROV is confirmed or suspected.'
The CDC says further investigation is required to determine the frequency of vertical transmission and whether the timing of OROV infection during pregnancy increases the risk for an adverse outcome, such as microcephaly.
Previously, the CDC issued a Travel Health Advisory stating pregnant women should reconsider non-essential travel to Oropouche-risk areas such as Brazil and Cuba. But, if travel is unavoidable, these travelers should strictly follow Oropouche's prevention recommendations.
As of October 9, 2024, Zika vaccine candidates are conducting clinical research, but Oropouche vaccines have yet to launch human clinical trials.
Our Trust Standards: Medical Advisory Committee