Next Season's Flu Shots Will Be Different
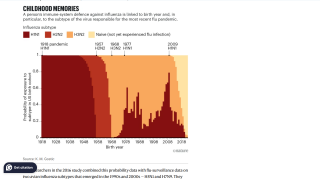
In the United States, influenza is a significant health concern each year, affecting millions of people and leading to hospitalization and death for many.
Flu vaccines have been recommended for over five decades and have been proven to lower the risk of flu and its severe complications in those who receive it.
The composition of U.S. flu vaccines is reviewed annually in time for newer flu vaccines to be manufactured each year.
On March 5, 2024, the U.S. FDA's Vaccines and Related Biological Products Advisory Committee (VRBPAC) met to discuss and recommend the viruses for the next flu season's vaccines.
The VRBPAC recommended that all 2024-2025 U.S. flu vaccines be three-component (trivalent) vaccines and include an influenza A(H1N1), an A(H3N2), and a B/Victoria-lineage vaccine virus.
This decision was made because influenza B/Yamagata viruses are no longer circulating and have not been detected in global surveillance after March 2020.
Therefore, its inclusion in next season's flu vaccines is no longer warranted.
Initial news from vaccine producers has been very positive, with the intent to deliver the trivalent flu shots on time in 2024.
These types of vaccine changes have been made for many years.
From 1978-1979 through 2012-2013, flu vaccines were trivalent. But, quadrivalent flu vaccines became available in the U.S. during the 2013-2014 flu season.
The newer vaccines contained a fourth component—a second influenza B virus—to protect against both influenza B virus lineages.
As of March 2024, about 158 million quadrivalent flu vaccines had been distributed during the 2023-2024 season.
Furthermore, government agencies have review processes to determine if the flu vaccines, egg, cell, or nasal-based vaccines actually deliver virus protection.
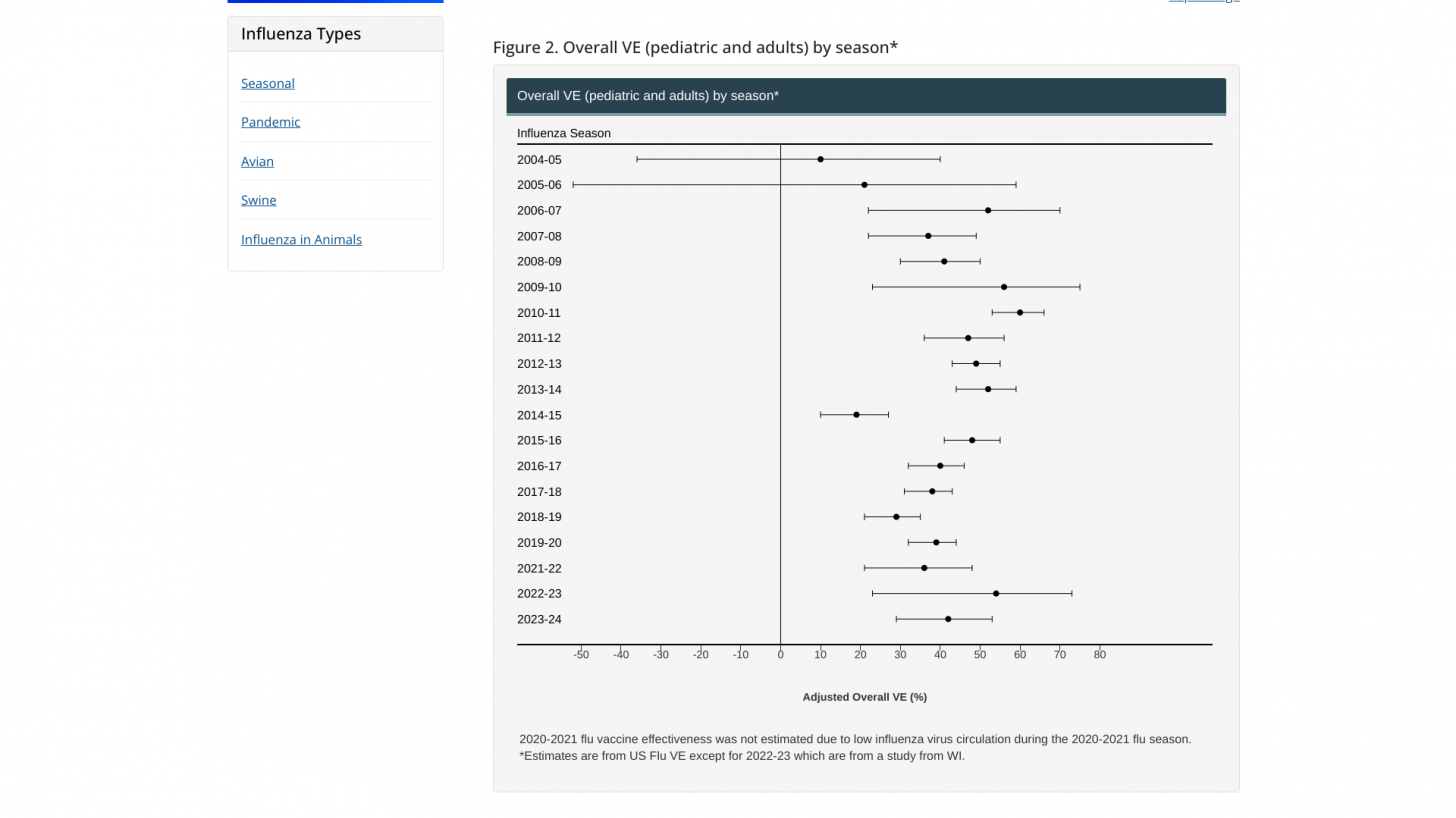
Regarding flu shot protection, on February 28, 2024, Aaron M. Frutos, Ph.D., MPH, presented to the U.S. CDC's Advisory Committee on Immunization Practices (ACIP), confirming four networks evaluate vaccine effectiveness (VE) against laboratory-confirmed influenza for children, adolescents, and adults in the outpatient and inpatient settings.
According to the ACIP, the aggregated interim VE for this season's flu vaccines is about 50%. The final data is expected during a later CDC meeting.
Our Trust Standards: Medical Advisory Committee