$4.8 Million Funds Japanese Encephalitis Virus Vaccine Patch

Vaxxas today announced that the Coalition for Epidemic Preparedness Innovations (CEPI) approved the progression of a $4.8 million program to develop heat-stable, dried-formulation mRNA vaccines delivered using Vaxxas’ needle-free high-density microarray patch (HD-MAP).
The Vaxxas HD-MAP is comprised of thousands of microscopic projections molded into a small patch. Each microprojection is coated with a small dose of vaccine in a dried formulation.
Announced on January 22, 2025, Vaxxas will partner with SK bioscience in this next phase of the program, advancing the company’s mRNA vaccine for Japanese Encephalitis Virus (JEV) on Vaxxas’ HD-MAP towards a Phase I clinical study.
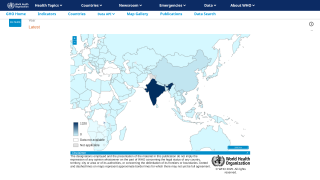
In late 2024, several JEV cases were confirmed in various Asian and Western Pacific Ocean countries.
Vaxxas expects the development work performed with the JEV vaccine candidate to be transferrable across all mRNA vaccine antigens delivered by LNPs, providing a platform approach that can be advanced to human trials.
David L. Hoey Vaxxas, CEO and President, commented in a press release, "With compelling proof-of-concept results in hand, we’re excited to have CEPI’s commitment to advance to the next stage of development."
"We’re equally excited to be working with SK bioscience and its JEV mRNA vaccine on this program to realize the promise of our HD-MAP technology to move the world closer to a commercially available, thermostable patch-based mRNA vaccine.”
This program was funded by CEPI in 2023 as part of its aim to improve the thermostability, and therefore equitable access, of mRNA vaccines.
This program is Vaxxas’ second collaboration with SK bioscience. The companies are also working on a program funded by Wellcome to advance the development of an HD-MAP/Typhoid conjugate vaccine candidate.
Our Trust Standards: Medical Advisory Committee