Orthopoxvirus (Mpox) Antiviral Approved in Japan

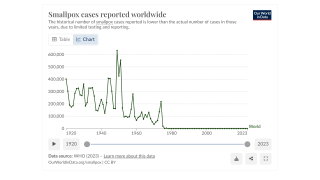
SIGA Technologies announced that its antiviral treatment TEPOXX (tecovirimat 200 mg capsules, TPOXX) had received regulatory approval in Japan for the treatment of smallpox, mpox, cowpox, as well as complications following smallpox vaccination in adults and pediatric patients weighing at least 13 kg. TEPOXX is the first antiviral therapy approved for the treatment of orthopoxviruses.
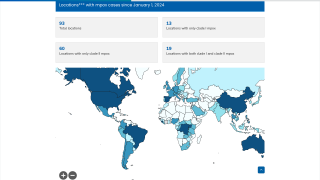
As of January 2, 2025, SIGA has delivered an order of TEPOXX to help build Japan’s strategic national stockpile.
TEPOXX is a highly targeted small-molecule antiviral that inhibits the VP37 protein found on the surface of all orthopoxviruses. By preventing the virus from exiting infected cells, TEPOXX slows the spread of the infection, enabling the immune system to clear the virus.
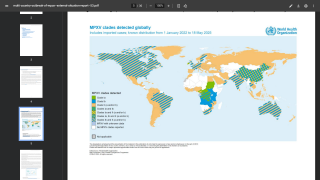
TPOXX is approved in the U.S. and Canada for the treatment of smallpox. In the European Union and the United Kingdom, marketed as Tecovirimat-SIGA, it is approved for treating smallpox, mpox, and cowpox and to treat complications following smallpox vaccination.
The Japanese approval is based on data from 15 clinical trials of oral TEPOXX in over 800 healthy volunteers, including a pivotal repeat-dose phase 1 pharmacokinetics (PK) trial involving 20 healthy volunteers conducted in Japan. TEPOXX has also been studied in NHPs infected with the variola virus, which causes smallpox, where TEPOXX demonstrated improved survival and reduced lesions.
In 2024, the U.S. NIH announced unsatisfactory results regarding mpox usage.
Our Trust Standards: Medical Advisory Committee