Clinical Trial Market Could Reach $80 Billion
During the recent pandemic, many scientists were stunned by how fast government agencies authorized new vaccines. Traditionally, it took about eight years for a vaccine to progress through the various stages of clinical trials to obtain market approval.
Many scientists were amazed by the speed at which new vaccines were authorized by government agencies during the recent pandemic.
Traditionally, it took about eight years for a vaccine to progress through the various stages of clinical trials to obtain market approval.
However, new research published on March 21, 2024, suggests that the clinical development of innovative medicines and vaccines will continue to accelerate.
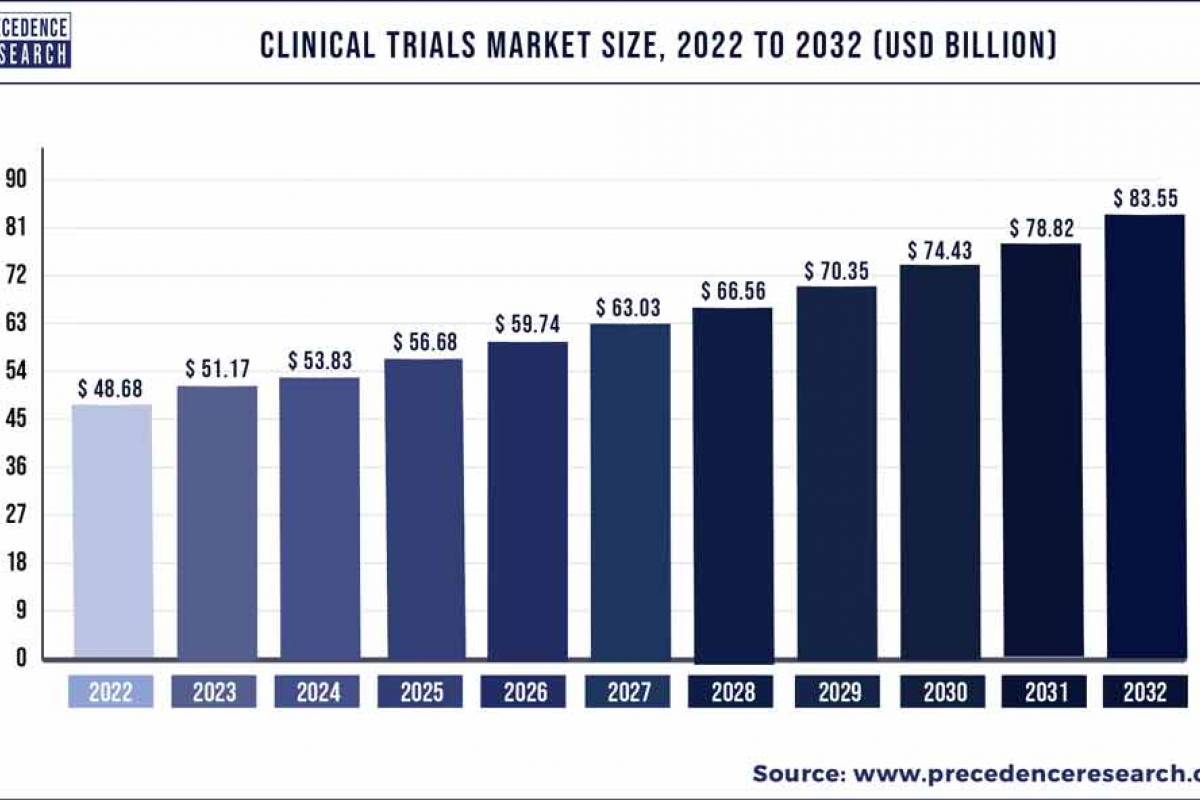
Precedence Research Pvt. Ltd. reported today that the global clinical trials market size was valued at $48.68 billion in 2022 and is predicted to reach about $83 billion by 2032. Clinical trial research was valued at $24.61 billion in the US sub-market in 2022.
The vaccine sub-market reached $102.9 billion in 2023 and is forecasted to expand by 47% to $35.1 billion by 2030.
Furthermore, there is plenty of investor interest in funding new, innovative vaccines.
In the past ten years, companies with infectious disease vaccine programs received 3.4% of the total ($6.5 billion) venture capital raised for biopharmaceutical companies.
Moreover, the promise of personalized vaccines tailored to subpopulations may disrupt the one-size-fits-all vaccination model, further expanding the need for clinical trial research.
Our Trust Standards: Medical Advisory Committee







