New Polio Vaccine Available in 35 Countries
In late 2023, the World Health Organization issued its first-ever prequalification approval for a vaccine under its Emergency Use Listing (EUL) regulatory pathway, the novel oral polio vaccine type 2 (nOPV2).
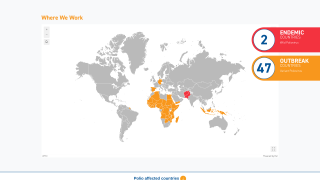
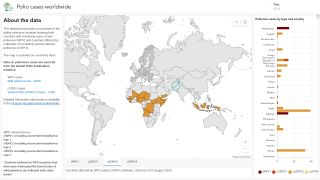
Since this next-generation vaccine rollout began in March 2021, the Global Polio Eradication Initiative (GPEI) reported about 1 billion nOPV2 doses have been administered across 35 countries, protecting children against disease.
WHO prequalification will enable additional countries to access the vaccine in response to outbreaks of type 2 variant poliovirus (cVDPV2).
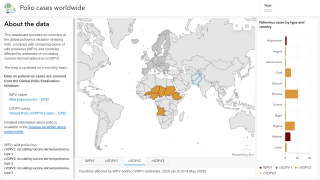
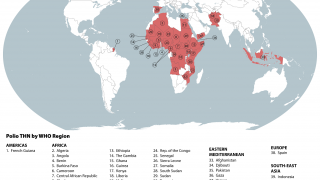
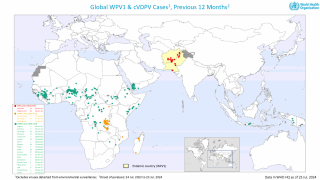
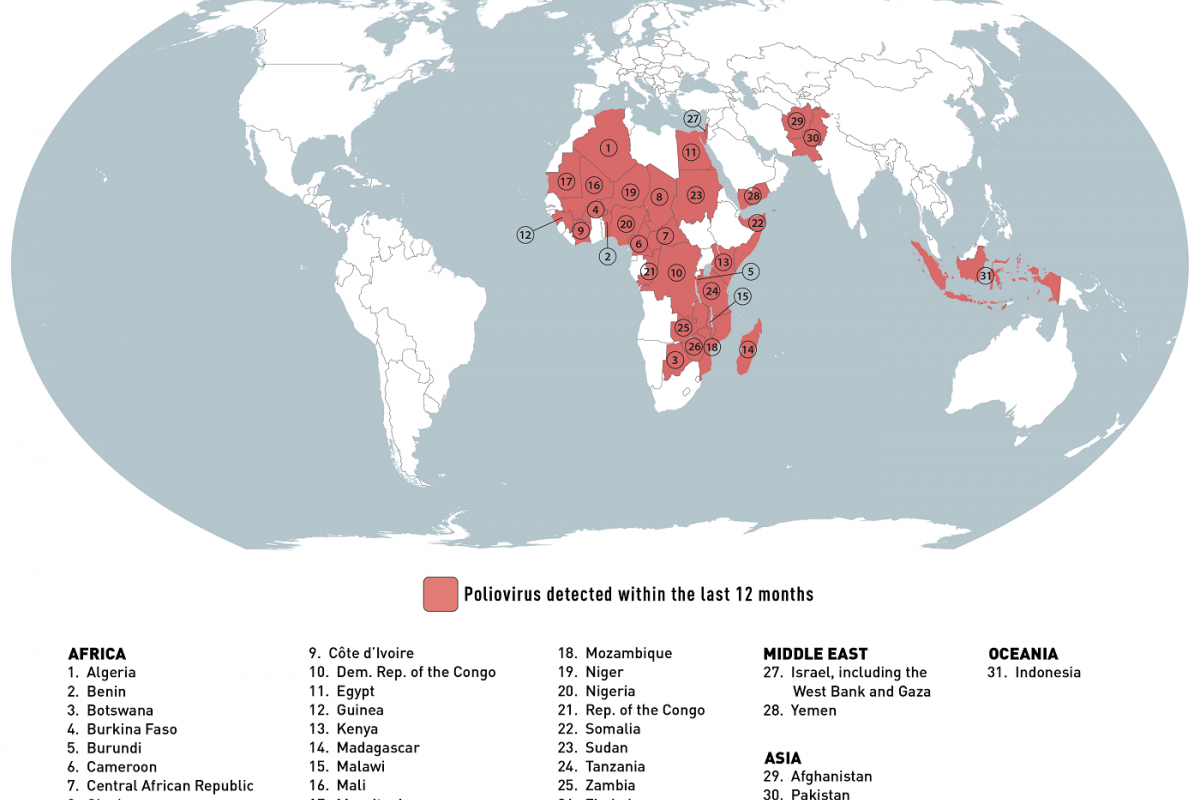
As of January 3, 2024, the GPEI reported that 325 cases of cVDPV2 had been confirmed in 2023, compared to 689 cases in 2022.
While nOPV2 has played a vital part in this reduction, its success, like any polio vaccine, depends on the ability to implement high-quality immunization campaigns that reach every child rapidly, says the GPEI.
"This is a historic milestone for polio eradication and public health," commented WHO Director-General Dr Tedros Adhanom Ghebreyesus in a press release.
"Novel oral polio vaccine type 2 has blazed a trail for other new vaccines that address critical health emergencies, and its use demonstrates the utility of the EUL mechanism in helping to rapidly get new products to where they're needed most."
The nOPV2 vaccine is genetically more stable than existing oral polio vaccines, with a lower risk of reversion to neurovirulence. In addition, this nOPV2 vaccine produces a gut reaction that stops virus transmission using a more stable version of the OPV, which is much less likely to cause paralysis.
The WHO's EUL is reserved for using yet-to-be-licensed vaccines, medicines, and diagnostic tools during public health emergencies like polio outbreaks.
As of January 10, 2024, the nOPV2 vaccine is unavailable in the United States.
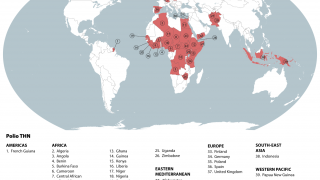
According to a recent U.S. CDC Travel Health Notice, over 30 countries reported polio outbreaks in 2023.
Our Trust Standards: Medical Advisory Committee