$1.2 Million Empowers Under-the-Tongue Vaccine Film Delivery

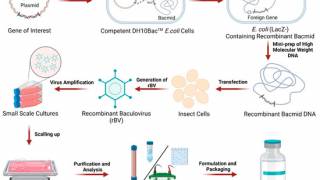
The Coalition for Epidemic Preparedness Innovations (CEPI) recently announced it had partnered with Jurata Thin Film, Inc. to advance development of thermostable under-the-tongue vaccine films as a needle-free vaccine delivery platform.
On December 5, 2023, CEPI confirmed that it will provide up to an initial $1.2 million to support Jurata's proprietary innovative formulation platform, which, if shown to be successful, could help expand access to vaccines in underserved regions and advance the global response to future emerging infectious disease outbreaks.
CEPI's initial funding will support optimizing the composition and process of creating thin films and preclinical studies.
Under the agreement with CEPI, Jurata will create vaccine films to remain stable at 2-8 degrees, 25 degrees, and 40 degrees.
Jurata will optimise the composition of the films by testing various buffers, pH, stabilizers, sugars, salts, and different drying parameters and assessing how this affects vaccine stability and delivery.
Jurata aims to improve vaccine accessibility by stabilizing the 3D structure of mRNA-containing lipid nanoparticle vaccine materials, provided by Quantoom Biosciences, part of Univercells, into a thin thermostable film, thereby removing frozen storage needs.
The vaccine films are also lightweight and compact, simplifying the transportation process and potentially allowing for more doses to be shipped at any one time compared to current needle-and-syringe distribution.
Dr. Irnela Bajrovic, Chief Scientific Officer, Jurata, commented in a press release, "Our stabilising formulations have the potential to facilitate global access to mRNA vaccines, and our thin film delivery platform could make vaccine administration far easier than needle-and-syringe injections."
"We are grateful to CEPI for supporting our innovative technology and look forward to working with Quantoom to show the breadth of mRNA vaccines our technology can stabilize and deliver."
This is the fourth partner to be announced as part of CEPI's Call for Proposals for thermostable vaccine manufacturing innovations, announced in January 2022.
Thermostable vaccines are also identified as a preferred vaccine characteristic by the World Health Organization.
Our Trust Standards: Medical Advisory Committee