U.K. Advice's Meningococcal Group B Vaccination to Prevent Gonorrhea

The U.K.'s Joint Committee on Vaccination and Immunisation (JCVI) today advised the government of a new, routine targeted vaccination program to prevent gonorrhea.
On November 10, 2023, the JCVI announced it had agreed that a targeted program should be initiated using the 4CMenB (Bexsero®) vaccine for the prevention of gonorrhea in those who are at most significant risk of infection.
The JCVI advice is that Bexsero should be offered on an opportunistic basis through specialist sexual health services, which have the experience in assessment and identification of those who are at increased risk of infection with bacterial sexually transmitted infections (STI).
The U.S. Food and Drug Administration (FDA) initially approved Bexsero (Meningococcal Group B Vaccine) for intramuscular injection in 2015.
In the U.S., Bexsero is generally available at health clinics and pharmacies.
As of November 2023, no vaccines are approved by the FDA or European Medicines Agency for preventing gonorrhea infection.
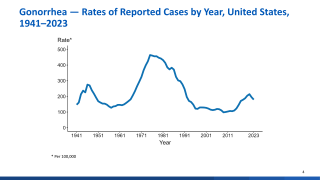
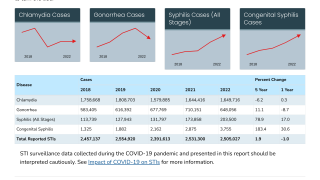
The U.S. Centers for Disease Control and Prevention (CDC) reported about 710,000 cases of Gonorrhea in 2021, making it the second most common STI in the U.S.
In addition, the CDC announced on April 11, 2023, that gonorrhea rates increased by more than 4%, reaching 710,151 in 2022.
Our Trust Standards: Medical Advisory Committee