Malaria Prevention Regimen Issued Another Patent

60 Degrees Pharmaceuticals Inc. today announced that the Canadian Intellectual Property Office issued a patent on using novel tafenoquine regimens for malaria prevention in malaria-naive individuals, and it will remain valid until December 2, 2035.
The Company was issued a similar U.S. patent in 2019.
Tafenoquine is the active molecule in the Company's U.S. Food and Drug Administration-approved regimen for malaria prevention, ARAKODA®.
ARAKODA, an oral tablet containing 100 mg of tafenoquine base, is an anti-malarial indicated for malaria prevention in individuals 18 years and older.
As of July 31, 2023, travelers or individuals at risk of contracting malaria are prescribed 2 x 100 mg tablets once per day for three days (the loading phase) before travel, 2 x 100 mg tablets weekly for up to six months during travel, then 2 x 100 mg in the week following travel.
Travelers from, and residents of, Canada and the United States, are usually malaria naive because they have not previously contracted malaria and thus lack immunity to the disease.
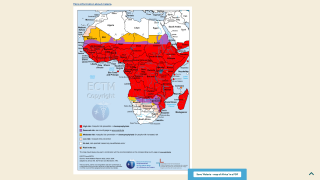
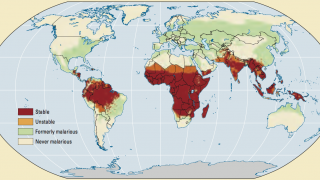
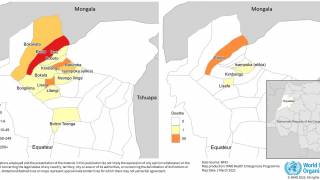
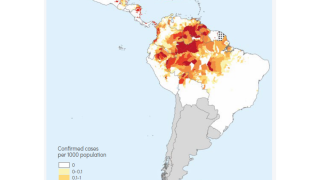
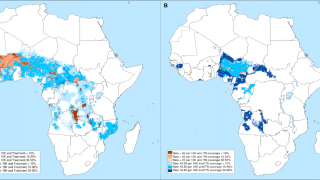
During July 2023, malaria outbreaks have been reported in Florida and throughout Central America in countries such as Costa Rica. And numerous African countries have confirmed malaria outbreaks.
The bite of an infective female Anopheles mosquito spreads malaria. The disease can cause fever, chills, and flu-like illness. If it is not treated, it can cause severe complications and death, says the U.S. CDC.
Typically, about 2,000 malaria cases are diagnosed in the United States yearly.
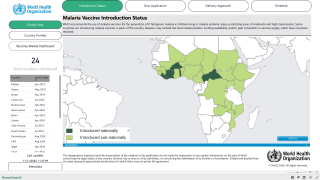
In Africa, there are two malaria vaccines currently being administered.
And on April 26, 2023, the United States Patent and Trademark Office issued 60 Degrees a patent covering the use of tafenoquine as a treatment for COVID-19 disease.
Our Trust Standards: Medical Advisory Committee