Nigeria Authorizes Innovative Malaria Vaccine

Prof Mojisola Christianah Adeyeye, Director-General of the Federal Republic of Nogeria's National Agency for Food and Drug Administration And Control (NAFDAC), today announced it granted registration approval for the R21/Matrix-M™ vaccine.
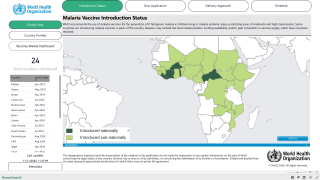
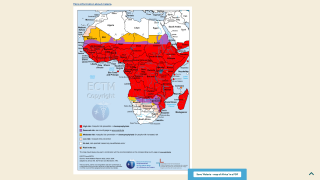
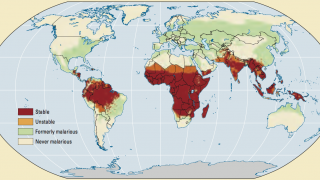
The NAFDAC approval on April 17, 2023, is essential since the WHO African Region continues to carry a disproportionately high share of the global malaria burden.
For example, in 2021, the Region was home to about 95% of all malaria cases and 96% of deaths.
And malaria is transmitted throughout Nigeria, with 97% of the population at risk of malaria.
Furthermore, the prevalence of malaria parasitemia in Nigerian children under five is about 23%.
The R21 was created by the University of Oxford Jenner Institute in England, is manufactured by Serum Institute of India Pvt. Ltd., and includes Novavax AB proprietary saponin-based Matrix-M adjuvant.
In addition to malaria, the U.S. CDC has issued various Travel Advisories regarding disease outbreaks in Nigeria.
Vaccine-preventable diseases such as yellow fever, measles, and polio are health risks when visiting Nigeria in 2023.
Our Trust Standards: Medical Advisory Committee