Philippines Authorizes Shingles Vaccine Candidate Clinical Study

Jiangsu Recbio Technology Co., Ltd. today announced that it had recently received approval from the Philippines Food and Drug Administration for an early-stage clinical trial for its novel adjuvanted recombinant shingles vaccine, REC610.
The phase 1 study will evaluate the safety and immunogenicity of REC610 in healthy adult subjects aged 40 and above.
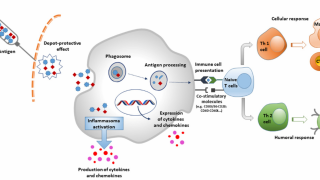
REC610 is equipped with a novel adjuvant BFA01 independently developed by the Company, which can promote the production of high levels of VZV glycoprotein E (gE)-specific CD4+ T cells and antibodies.
Preclinical studies have shown that REC610 has favorable immunogenicity and can induce high levels of gE-specific CD4+T cell responses and IgG antibody, and its immune response is non-inferior to the study's controlled vaccine Shingrix®.
Other shingles vaccine news is posted at PrecisionVaccinations.com/Shingles.
Our Trust Standards: Medical Advisory Committee