Don't Delay Shingles Vaccinations

The US Centers for Disease Control and Prevention (CDC) recently updated its Shingrix vaccine frequently asked questions (FAQ) webpage with guidance on shingles vaccination during the COVID-19 pandemic.
The CDC stated, 'Shingles vaccination is an essential preventive care service for older adults that should not be delayed or discontinued because of the COVID-19 pandemic unless a patient is suspected or confirmed to have COVID-19.'
The CDC recommends Shingrix to prevent shingles and its complications in adults 50 and older. You and patients should make every effort to ensure that two doses are administered within the recommended 2-6 month interval.
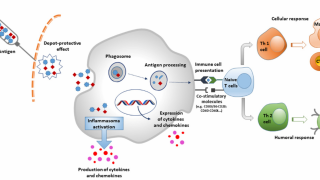
Shingrix is an adjuvanted recombinant zoster vaccine, consisting of the varicella-zoster virus glycoprotein E antigen and the AS01B adjuvant system, a proprietary adjuvant containing QS-21 and MPL with liposomes.
Furthermore, the CDC recommended on January 4, 2021, 'health care providers counsel patients about potential shingles vaccination side effects. This is especially important during the COVID-19 pandemic, since some shingles vaccination side effects may be similar to symptoms of COVID-19.'
Our Trust Standards: Medical Advisory Committee