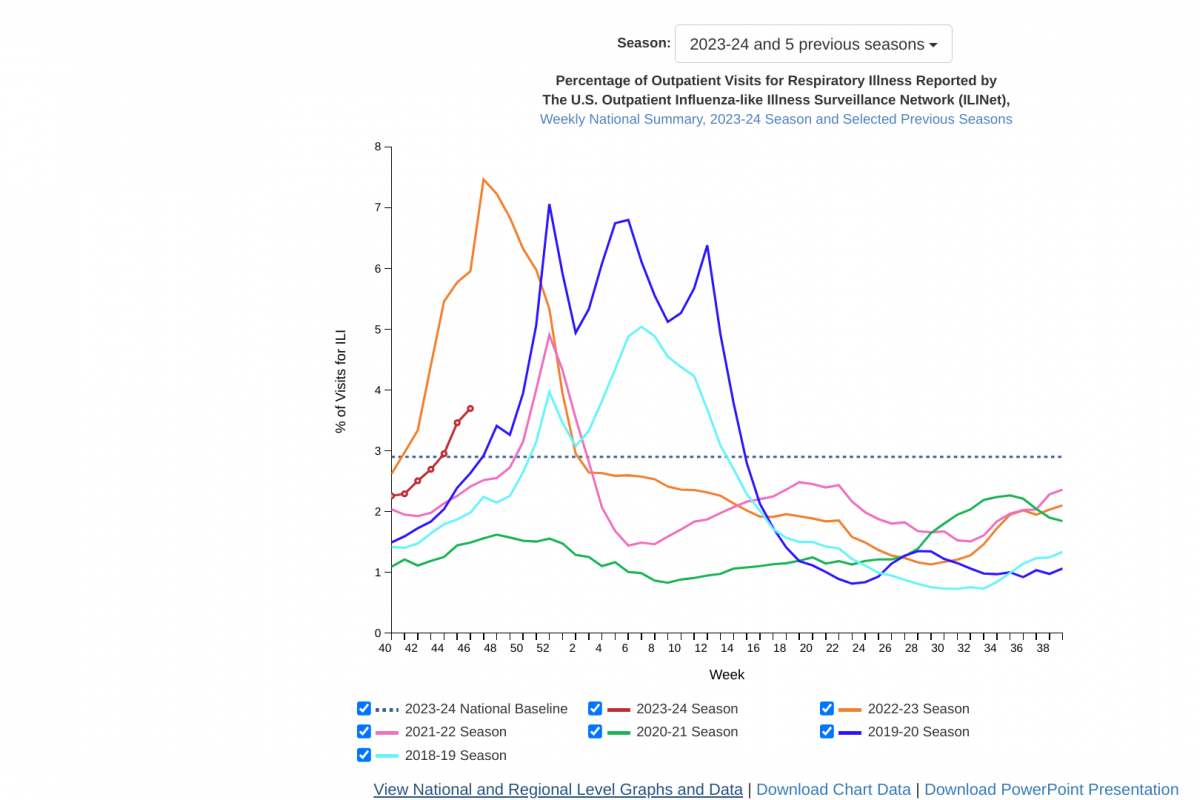
According to an updated U.S. Centers for Disease Control and Prevention (CDC) Influenza Surveillance Report, outpatient respiratory illness is above baseline nationally for the third week. And it is at or above baseline in seven of 10 HHS Regions.
As of November 27, 2023, Regions 1 and 8 (New England and Mountain) are at their region-specific outpatient respiratory illness baseline.
While Regions 2, 3, 4, 6, and 9 (New York/New Jersey/Puerto Rico/Virgin Islands, Mid-Atlantic, Southeast, South Central, and West Coast) are above their region-specific baselines.
Additionally, FluView data for week #46 indicates that influenza-related hospital admissions continue to increase.
Furthermore, the National Center for Health Statistics (NCHS) Mortality Surveillance data available on November 22, 2023, reports that 0.07% of the deaths during the week ending November 18, 2023, were due to influenza.
During week #46, there were 14 flu-related deaths.
During the 2023-2024 season, three influenza-associated pediatric deaths were reported to the CDC.
The CDC recommends an annual flu shot for most people over six months of age. Various flu shots are available at clinics and pharmacies in the U.S.
The CDC says that as of November 11, 2023, about 149 million flu vaccines had been distributed in the U.S.