SIGA Technologies, Inc. today reported financial results for the three and twelve months ended December 31, 2023.
"In 2023, SIGA had approximately $131 million in product revenues and approximately $84 million of pre-tax operating income," stated Diem Nguyen, Chief Executive Officer, in a press release on March 12, 2024.
"These financial results represent a significant increase over the 2022 financial results; product revenues increased 51% over the corresponding 2022 amount."
In 2023, SIGA had product sales of approximately $98 million in the fourth quarter of oral TPOXX® (tecovirimat, ST-246®), a novel small-molecule drug, to the U.S. Strategic National Stockpile ("SNS"); approximately $11 million of product sales of oral TPOXX to the U.S. Department of Defense, of which roughly $6 million was recognized in the fourth quarter; and approximately $21 million of international sales, of which approximately $12 million was recognized in the fourth quarter.
In the first two months of 2024, the Company delivered an additional approximately $15 million of oral TPOXX to the SNS, substantially completing the oral TPOXX order received in July 2023.
It also delivered an additional approximately $7 million of oral TPOXX to European countries and Canada.
Highlighting the continuing diversification of the Company's revenue base, the Company has received procurement orders for oral TPOXX from over 25 countries over the past two years.
As an example of the diversification trend, in October 2023, the Company reported the creation by the European Commission's DG HERA (Health Emergency Preparedness and Response Authority) of a joint procurement framework contract under which participating countries from the European Union and the European Free Trade Association can efficiently order oral TPOXX.
Under this mechanism, 13 countries ordered $18 million of oral TPOXX in the fourth quarter, and revenues were recorded for deliveries on substantially all of these orders in the fourth quarter or the first two months of 2024.
On March 12, 2024, SIGA's Board of Directors declared a special cash dividend of $0.60 per share, an increase of $0.15 per share from last year's special cash dividend.
In 2023, the Company paid a special cash dividend of $0.45 per share and repurchased approximately 1.7 million shares of its common stock.
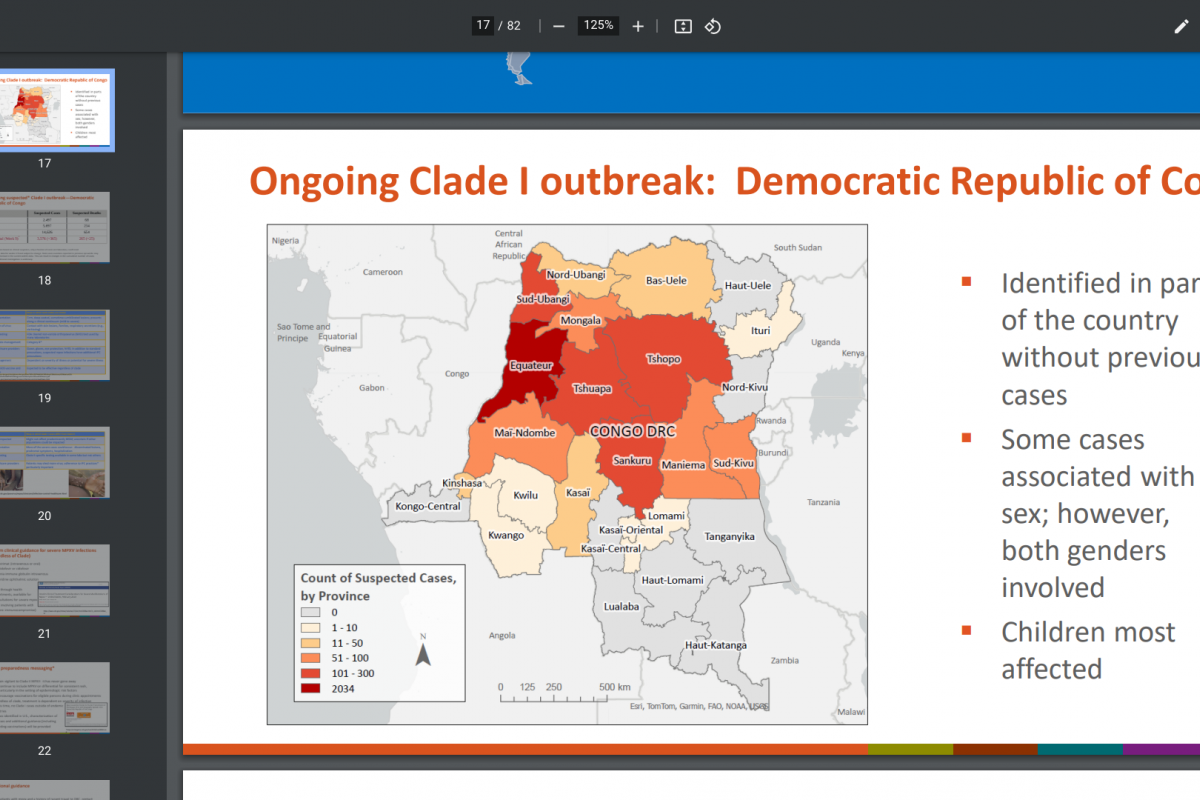
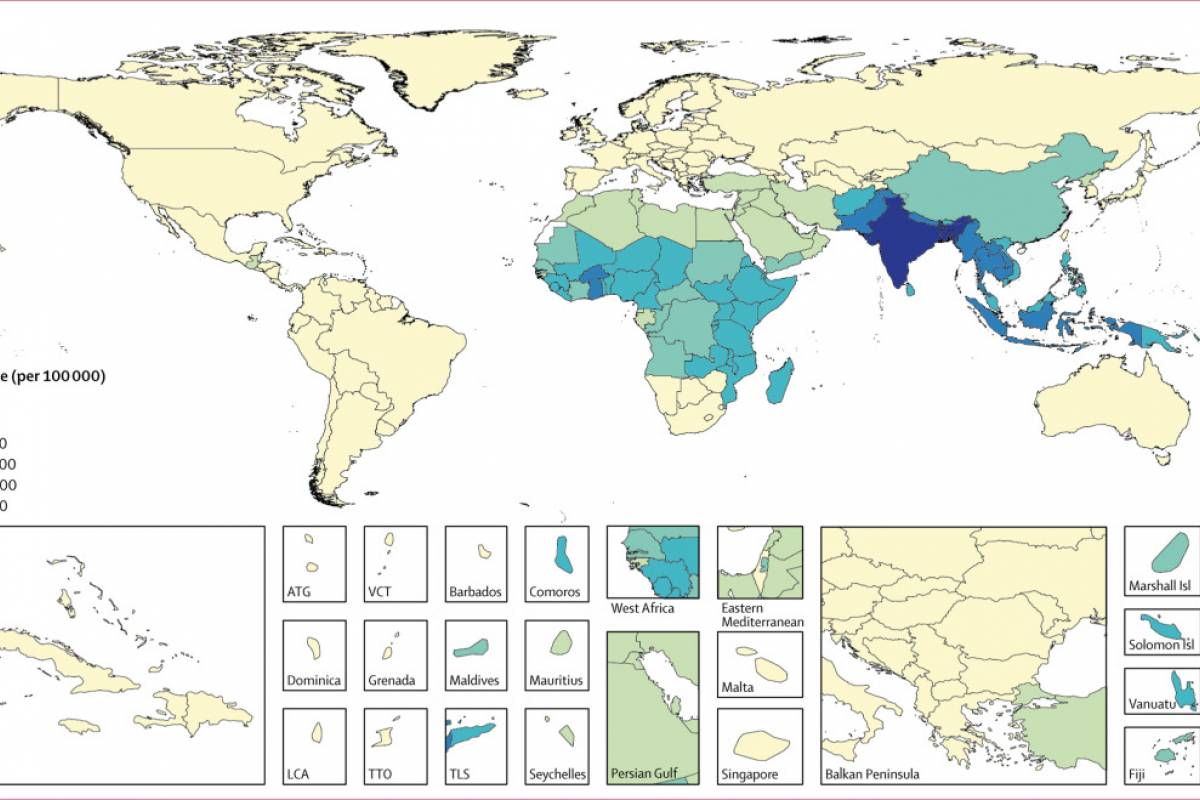
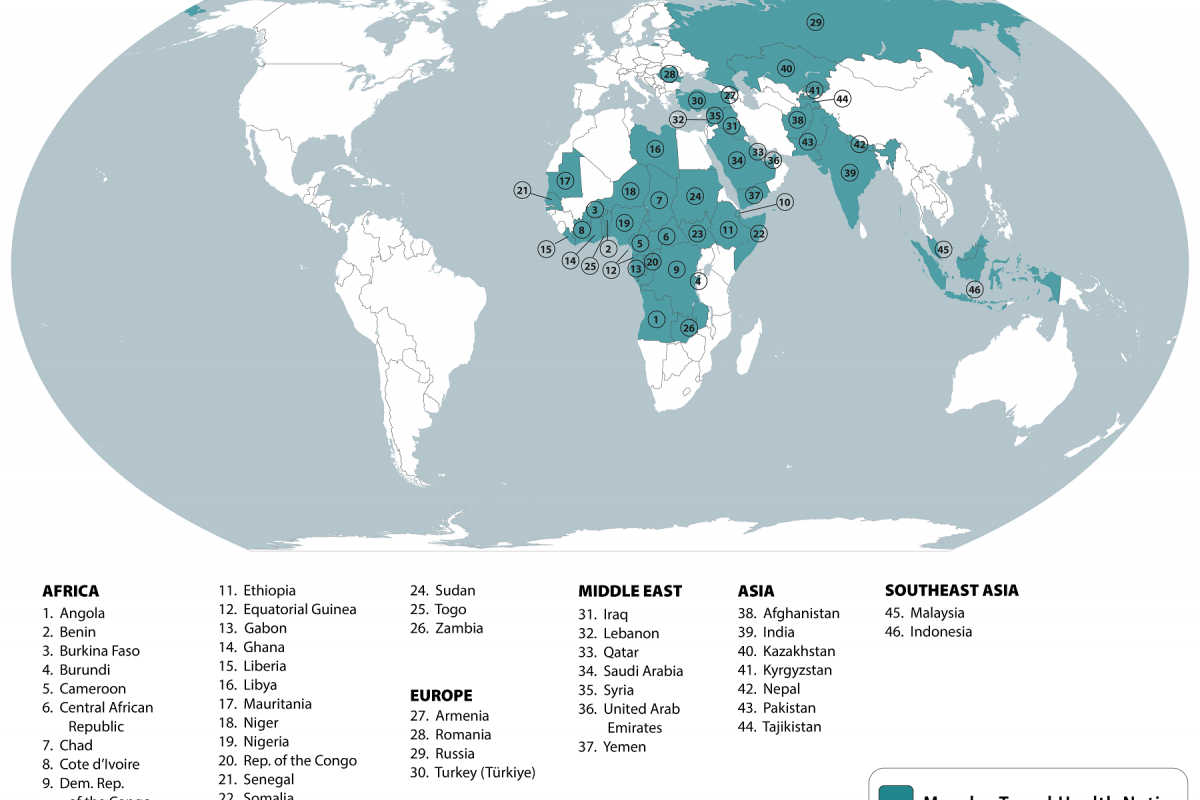
The first human case of Mpox was identified in the Democratic Republic of Congo (DRC) in 1970. The World Health Organization Mpox External Situation Report #31 confirmed on December 22, 2023, that it has received mpox case reports from 115 affected countries since May 2022.
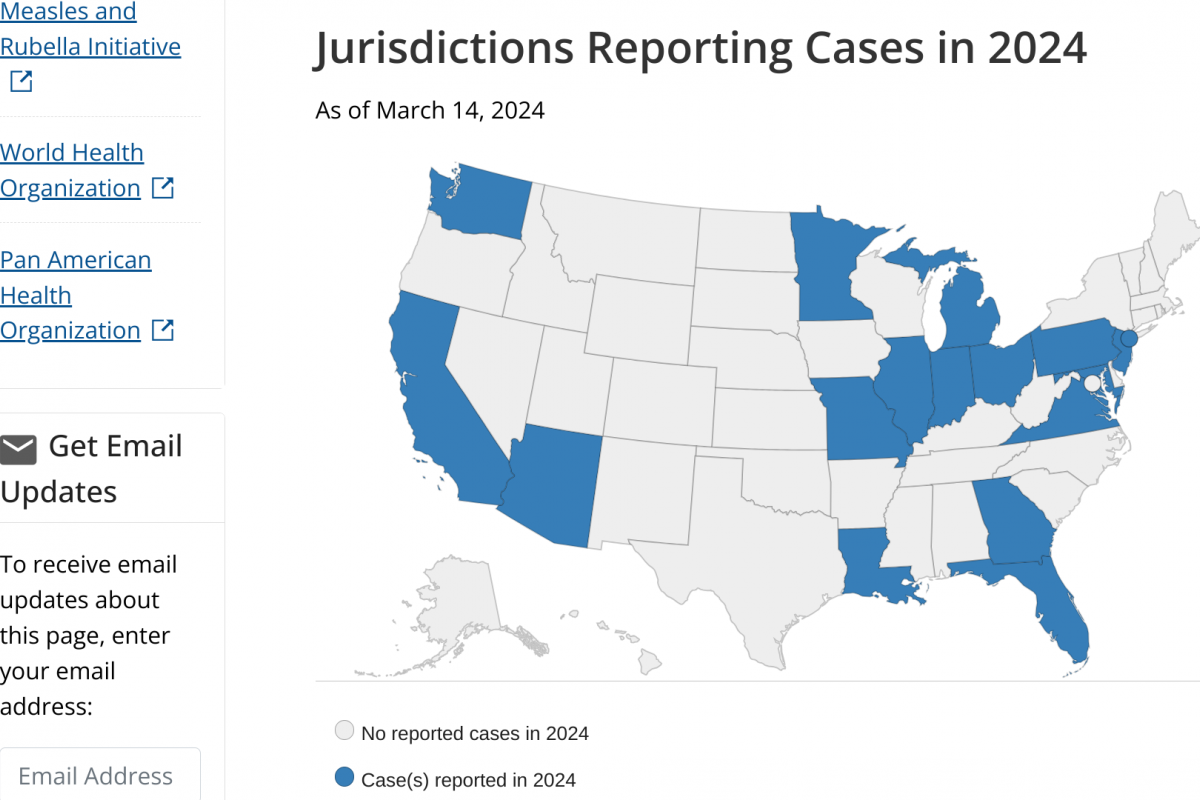
IN 2024, mpox outbreaks have been confirmed in various countries, including the United States and the DRC.
The U.S. CDC issued a Level 2 travel advisory health advisory in February 2024, alerting international travelers regarding mpox outbreaks in African countries.