Developed by Chengdu Weisjin Biomedical Technology Co., Ltd. (Wesjin Biotech), WGc-043 is an mRNA therapeutic cancer vaccine that recently received IND approval from the U.S. Food and Drug Administration (FDA).
According to public information from the FDA, WGc-043 Injection has been approved for two categories of indications: one is for adult patients with Epstein-Barr virus-positive advanced solid tumors who have undergone second-line systemic treatment.
The second indication is for adult patients with relapsed or refractory virus-positive hematoma.
This achievement, announced on May 9, 2024, marks the world's first approval of an Epstein-Barr virus (EBV)- related mRNA therapeutic cancer vaccine.
Once successfully launched, WGc-043 will provide a new treatment option for patients with advanced EB virus-positive solid tumors and hematologic malignancies.
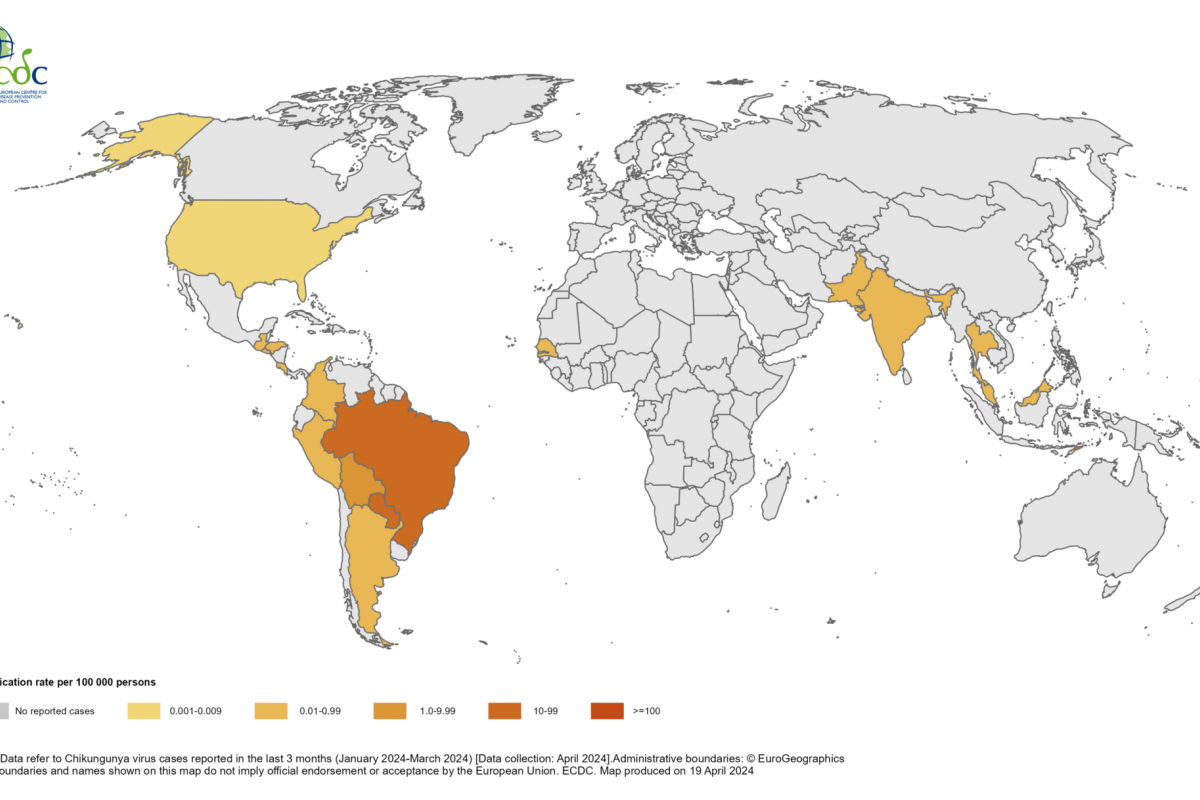
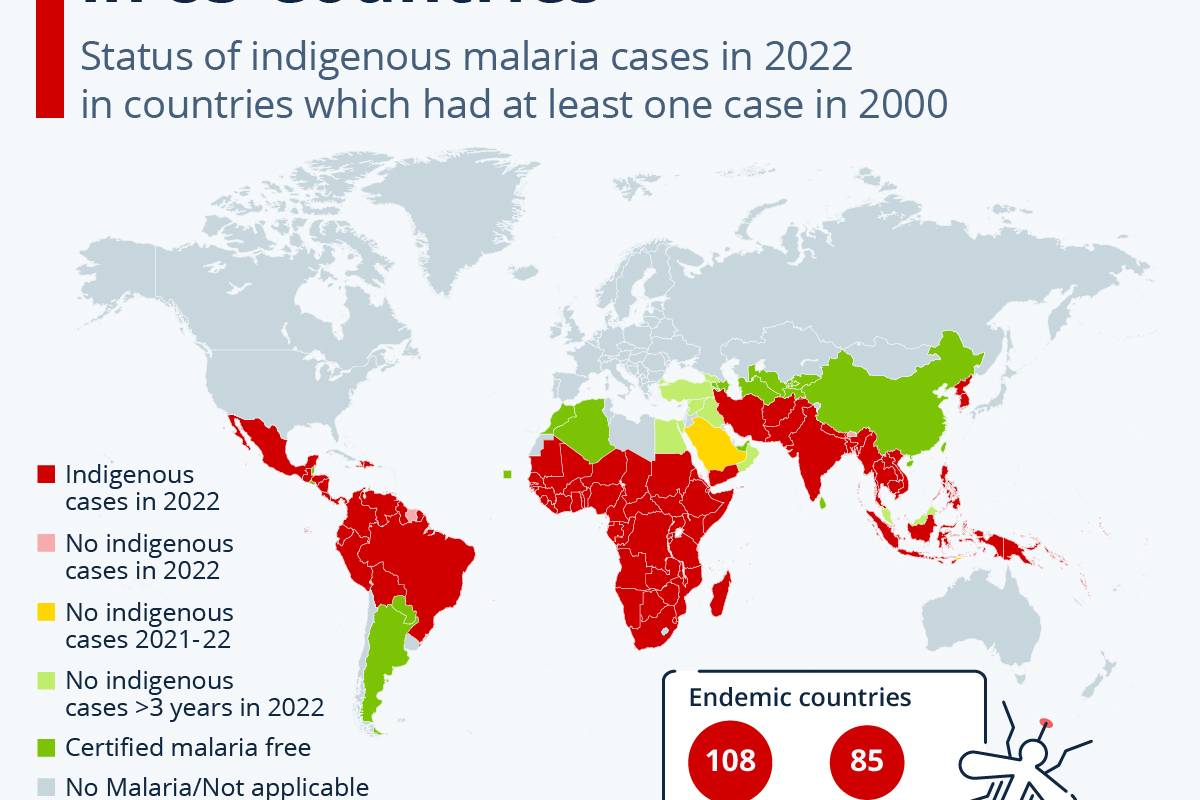
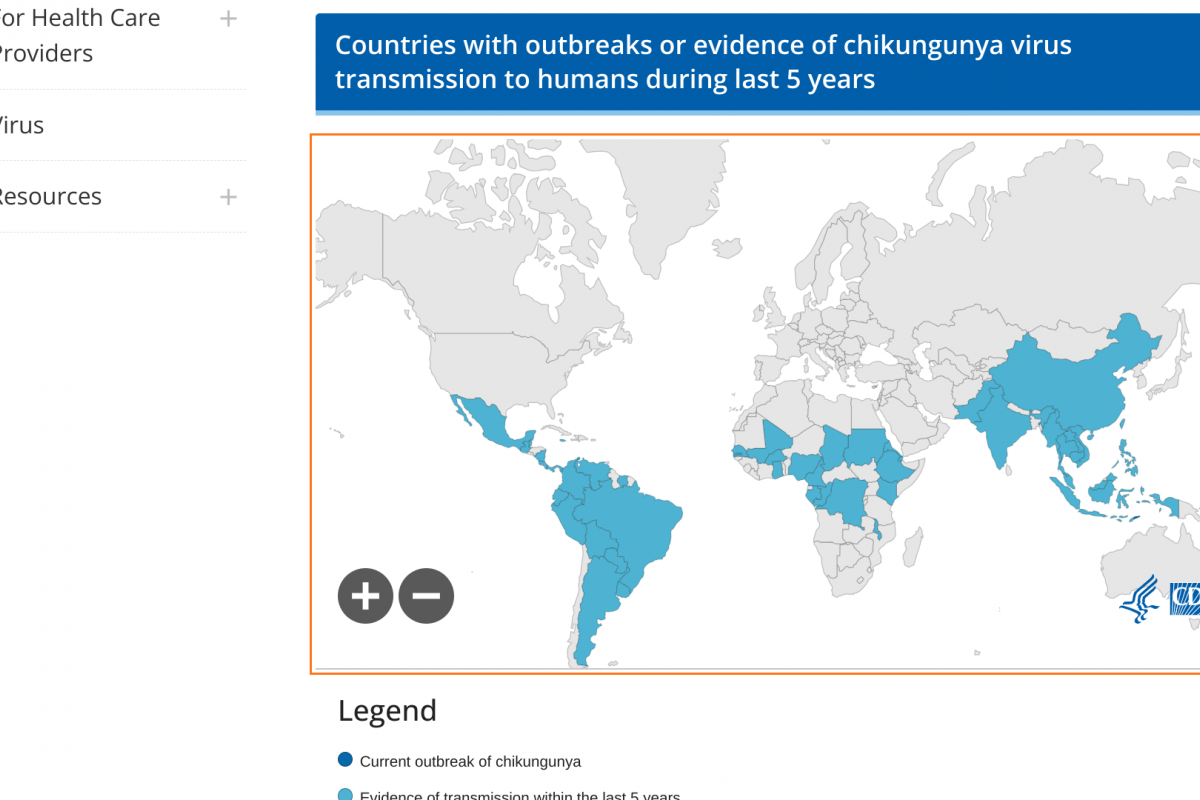
EB virus is highly correlated with more than ten malignancies, including nasopharyngeal carcinoma, natural killer T-cell lymphoma, gastric cancer, lung cancer, liver cancer, esophageal cancer, breast cancer, cervical cancer, and autoimmune diseases such as multiple sclerosis and systemic lupus erythematosus.
According to a press release on May 10, 2024, WGc-043 shows promising efficacy, low toxicity, broad applicability, efficient scalability, and cost-effectiveness.
The company says that WGc-043 has already completed investigator-initiated trials, demonstrating superior safety and efficacy compared to other publicly available mRNA therapeutic cancer vaccines.
Specifically, the technical features of WGc-043 also include the antigen being the most broad-spectrum and safe protein sequence.
The originally designed immune enhancer (IE) is introduced into the mRNA molecule, and the mRNA delivery carrier is independently developed and obtained
A new type of LNP authorized by US and European patents (the safety of this LNP: has been verified in clinical trials of 3 varieties).
These designs enable WGc-043 to activate the patient's own anti-tumor immunity and generate tumor-killing cytotoxic T cells, antigen-specific antibodies, and memory T cells in the body, which is equivalent to CAR-T. The combined anti-tumor effect of monoclonal antibodies can also prevent tumor recurrence, have more efficient anti-cancer effects, and be superiorly safe.
Weisjin Biotechnology has filed over 60 invention patents, including the patent for ionizable lipids, which has been authorized by China, the United States, Europe, and other countries and regions.
As of May 12, 2024, no date has been announced regarding WGc-043's availability in the United States.