The Coalition for Epidemic Preparedness Innovations (CEPI) and Bavarian Nordic A/S today announced a partnership to advance the development of Bavarian Nordic's Modified Vaccinia Ankara (MVA-BN®) vaccine (JYNNEOS®, IMVAMUNE®, IMVANEX®) for children in Africa.
On May 30, 3034, CEPI stated it awarded $6.5 million to support a Phase 2 clinical study evaluating the immunogenicity and safety of MVA-BN in children from 2 to less than 12 years of age compared to adults for the prevention of smallpox, mpox, and related orthopoxvirus infections.
This phase 2 clinical trial will be conducted in one or more African countries and is planned to be initiated later in 2024. Notably, the study will also generate evidence on the vaccine in endemic African populations and could potentially support regulatory approval of MVA-BN in endemic countries.
The new trial follows the publication of a continental plan by Africa CDC and African Ministries of Health to strengthen mpox preparedness and response efforts, as well as the World Health Organization's framework for enhancing the prevention and control of mpox.
"We now understand that children suffer disproportionately from mpox, a concerning and neglected disease that has spread rapidly in recent years," said Dr. Richard Hatchett, CEO of CEPI, in a press release.
"To address the risk children face in DR Congo (Clade I) and other areas where the disease is endemic, CEPI is supporting this important trial which will provide key mpox vaccine safety and immunogenicity data in children."
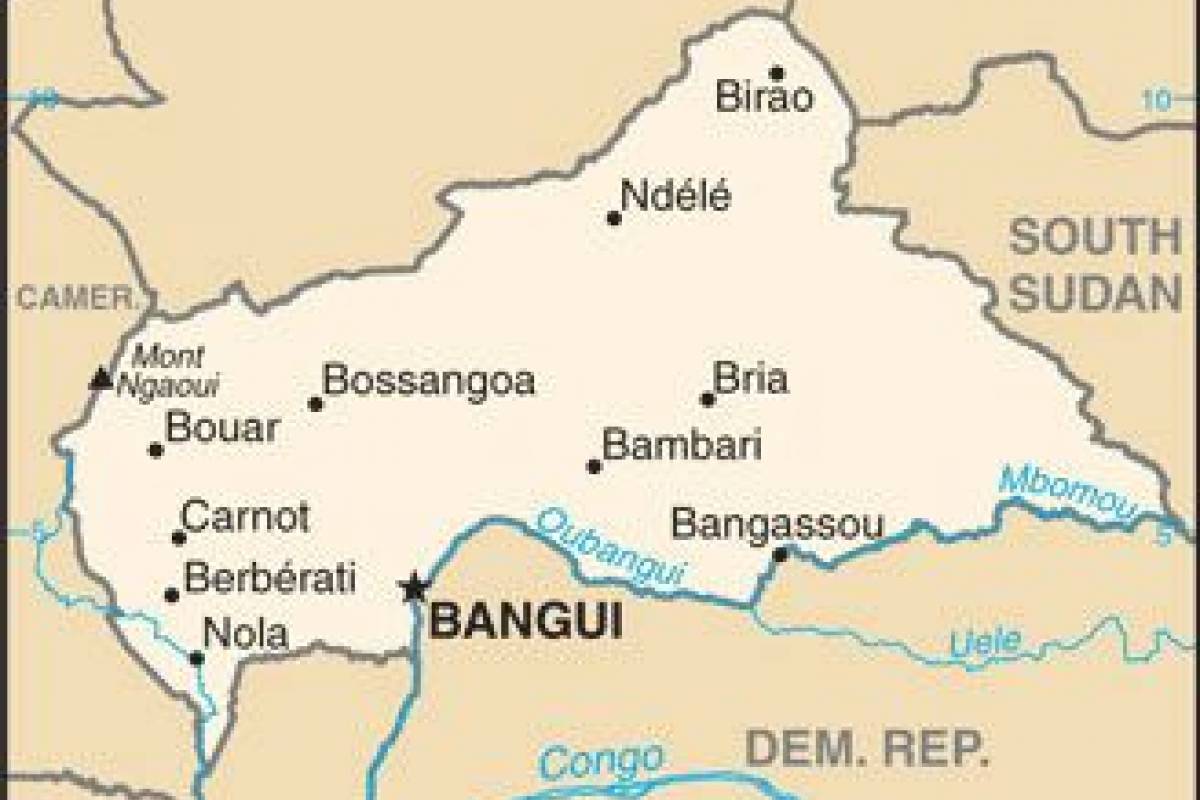
Over 6,500 mpox cases and 345 deaths have been reported in DR Congo in 2024, with children accounting for the majority of infections and deaths. Mpox was initially identified in the DR Congo in 1970.
In 2024, Mpox cases were also confirmed in the Congo, Cameroon, Central African Republic, and Liberia.
The mpox strain behind the current outbreak in Africa, known as Clade I, is estimated to be fatal in around 8-12% of cases. In the U.S., Clade II has been detected since May 2022.
MVA-BN or JYNNEOS is a non-replicating smallpox-mpox vaccine approved in the U.S., and is available at certain clinics and pharmacies in 2024.