Auro Vaccines LLC's Nipah Virus vaccine candidate HeV attachment G glycoprotein (HeV-sG-V) was recently found to induce antibodies within one month of vaccination, and the persistence afforded by two dosages suggests the vaccine candidate has the potential for reactive Nipal outbreak control and preventative use.
On May 30, 2024, results from a Phase 1 study funded by the Coalition for Epidemic Preparedness Innovations and published by The Lancet preprint evaluated a recombinant subunit vaccine consisting of a soluble version of HeV-sG-V for safety, tolerability, and immunogenicity. The highest response rates were among vaccinees receiving two administrations of the 100 mcg vaccine candidate 28 days apart.
As of June 6, 2024, several Nipah Virus vaccine candidates were conducting clinical research, but no approved Nipah vaccines were available.
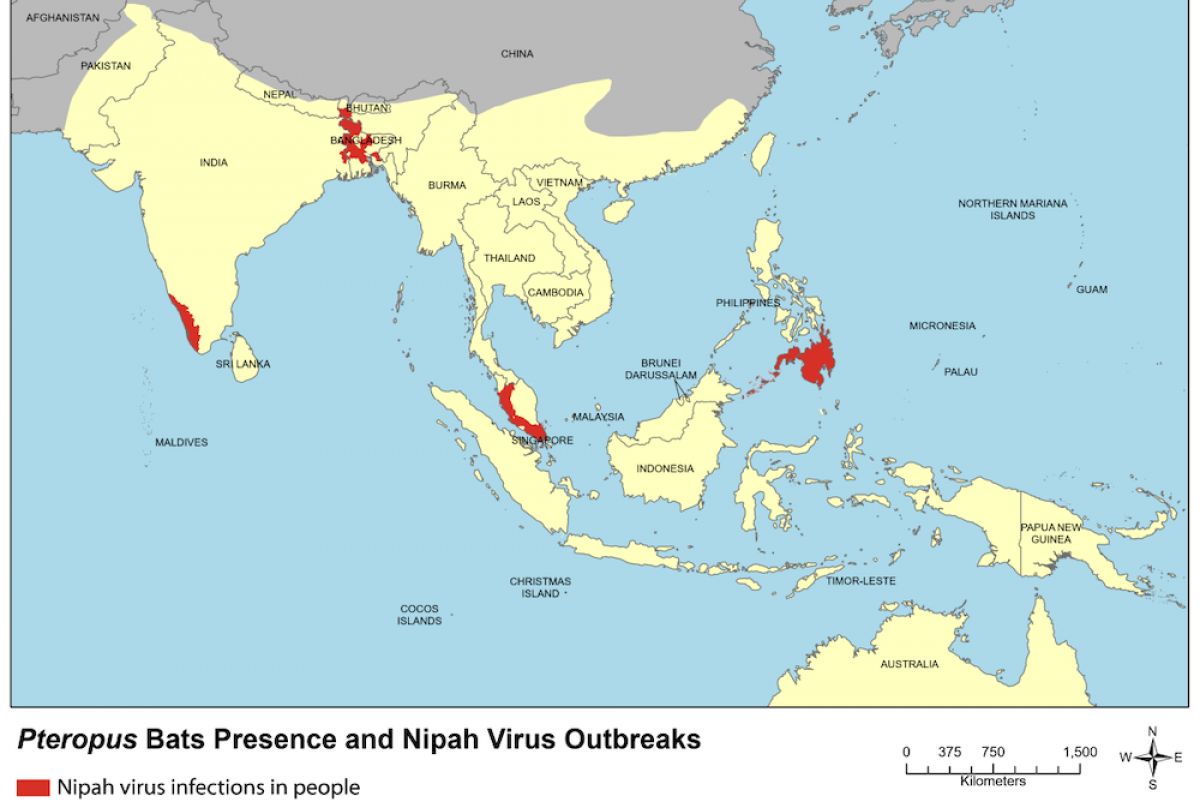
The World Health Organization says that Nipah was first discovered in Malaysia in the 1990s. This virus causes yearly outbreaks throughout South and Southeast Asia, with associated mortality rates of 40 to 75 %. Nipah infection is a zoonotic illness transmitted to people from animals such as bats.
The virus can also be transmitted through contaminated food or from person to person.
The WHO published a Technical Brief in early 2024 as an interim document to guide countries in planning for a Nipah virus event.
No cases of Nipah have been diagnosed in the U.S.