The Pan American Health Organization (PAHO) reported yesterday a significant increase in pertussis (whooping cough) cases in the Region of the Americas.
On July 22, 2024, the PAHO confirmed that 7,251 pertussis cases were reported in the United States in 2024, a 300% increase from last year.
Pertussis cases in Mexico are 242% higher than reported in 2023. Brazil and Peru are also reporting measurable case increases.
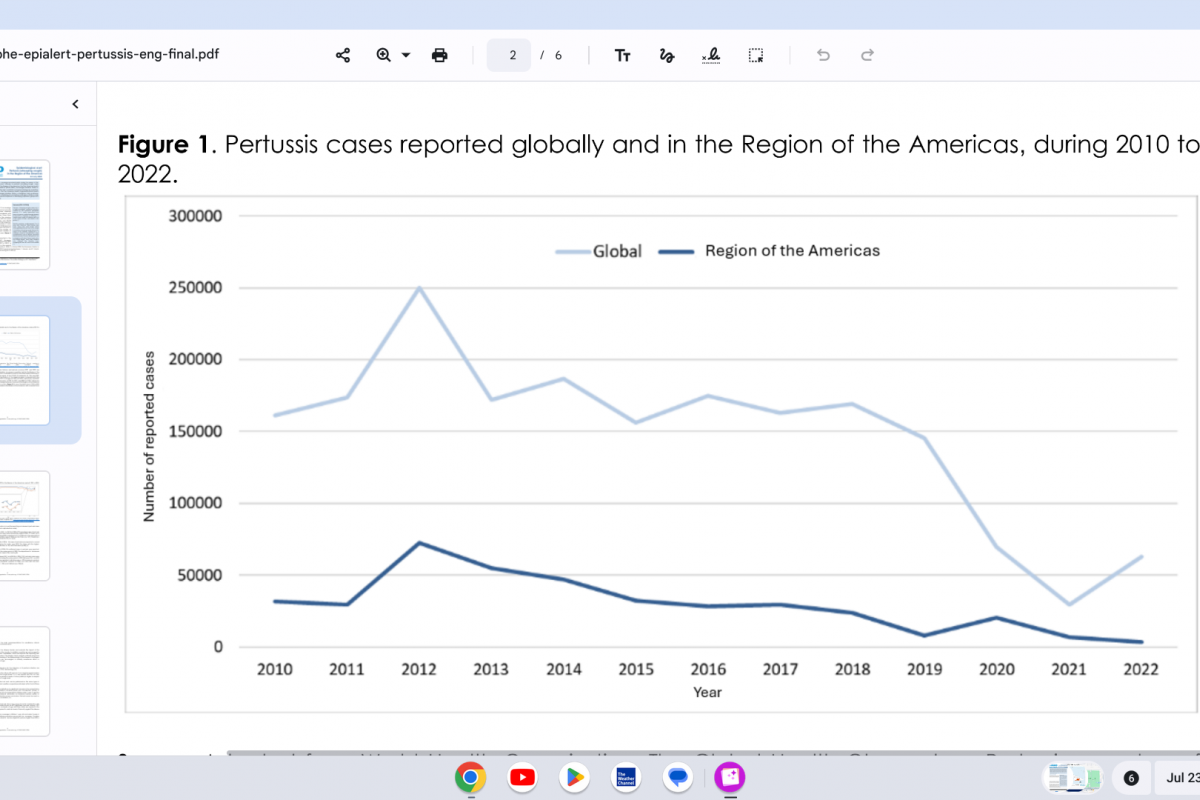
In the Region of the Americas, 2012 was the year with the highest number of cases reported during the decade, with 72,328 reported cases of pertussis. Since then, there has been a progressive annual decrease in the reported cases, reaching the lowest number reported in 2022, with 3,283 pertussis cases.
The first and third doses of diphtheria, tetanus, and pertussis vaccines (DTP1 and DTP3) are commonly used as tracers of immunization coverage. The coverage trend for both first and third doses has shown a significant decline.
The year 2021 was the lowest coverage year in the Region of the Americas compared with the previous 20 years. However, updated vaccine coverage data for 2023 reported a recovery of 90% for DTP1 and 88% for DTP3.