Visiting areas where Valley Fever is common can put any mammal at risk of infection from breathing in fungal spores. Over the past decade, Valley Fever fungus (coccidioidomycosis) has sickened thousands of healthy people.
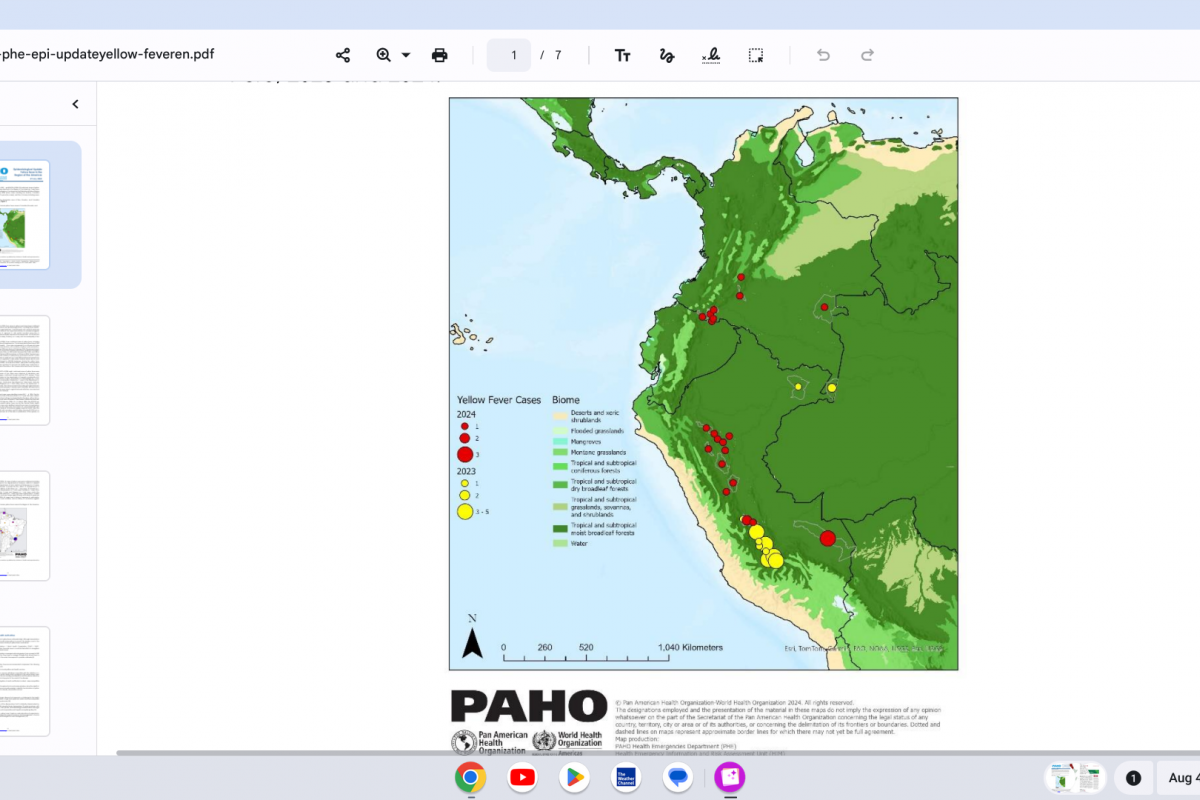
This fungus is found in regions like the southwestern United States, Mexico, and Central and South America.
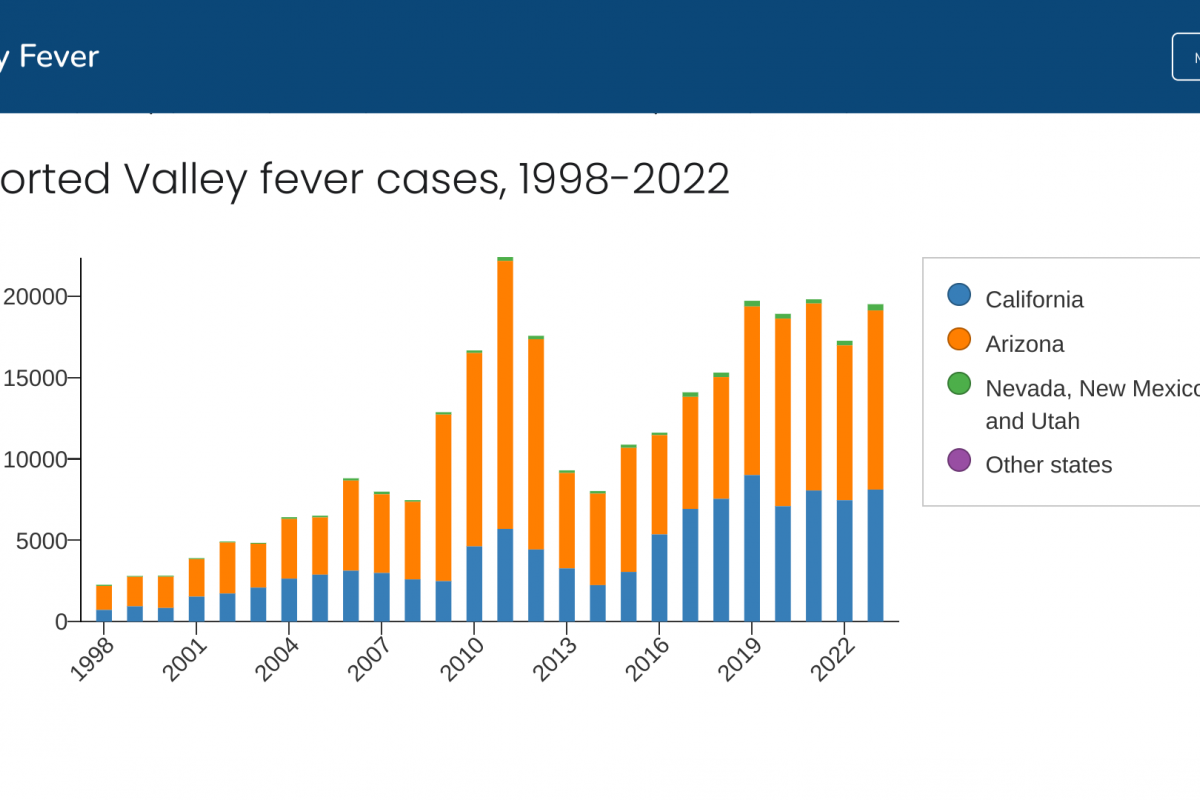
In 2022, U.S. states reported a total of 17,612 cases of Valley Fever to the Centers for Disease Control and Prevention.
To address this health risk, Anivive Lifesciences Inc. recently announced that the National Institute of Allergy and Infectious Diseases (NIAID has awarded (#75N93024C00009) worth up to $33M to support the development of a Valley Fever preventive vaccine.
The funding will address enabling activities, including additional manufacturing, formulation, extensive safety testing, and an IND submission, before completing a human Phase 1 clinical trial.
"Anivive is honored to receive this NIAID contract, which will greatly accelerate our efforts to commercialize a vaccine to protect people against Valley Fever," said Dr. Edward Robb, Anivive Lifesciences Chief Strategy Officer and Principal Investigator, in a press release on August 2, 2024.
"This collaborative effort has delivered a significant step forward in the field of vaccinology and holds the potential to be the first vaccine to prevent a serious systemic fungal infection common to humans and animals," said Robb.
As of August 5, 2024, details of the phase 1 study were pending.
Additionally, this program is supported by Valley Fever Center for Excellence at the University of Arizona College of Medicine for the non-clinical development, Recipharm for the contract manufacturing, Quigley BioPharma for vaccine development support, and Latham BioPharm Group, part of Sia Partners for the program management, financial compliance, quality assurance, and additional technical subject matter expertise.