Over the past two years, measles outbreaks have significantly increased in various countries, but not the United States.
However, the Texas Department of State Health Services (DSHS) recently published a Health Alert regarding two measles cases.
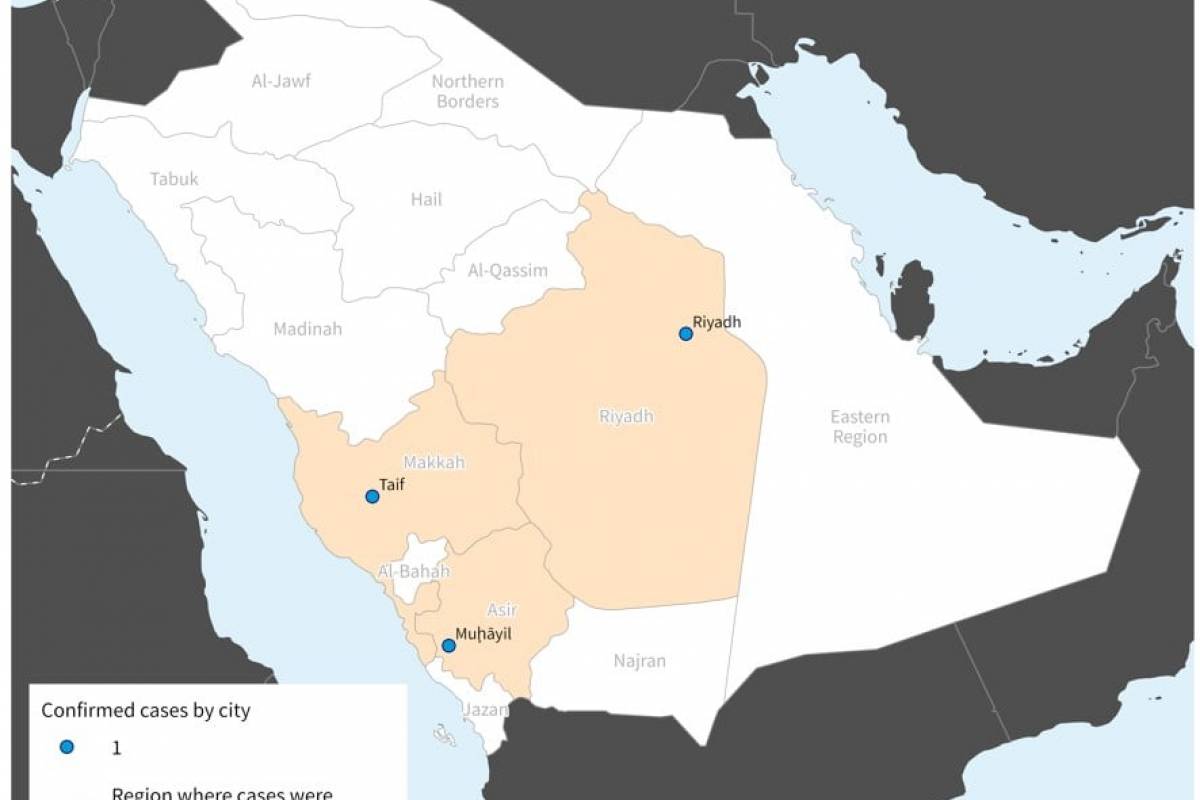
On August 29, 2023, DHSH confirmed the first measles case was diagnosed in June in a resident of Hood County.
The second was diagnosed in August in McCulloch County.
Neither adult patient had a history of travel to an area where measles is spreading, and no other risk factors for exposure were identified. At this time, public health officials do not suspect these cases are related.
Both cases had received one dose of the measles-mumps-rubella (MMR) vaccine.
The U.S. CDC says one dose of MMR vaccine is 93% effective against measles, while two doses offer 97% protection.
Before vaccine introduction, annual measles incidence in Texas peaked at 85,862 in 1958. In 2019, Texas experienced a measles outbreak of 23 cases.
In 2019, research revealed three Texas counties, Harris, Tarrant, and Travis, were at risk for measles outbreaks. The study found two main factors: vaccine refusal rates, and the number of travelers from other countries.
As of early August 2023, the CDC reported 19 measles cases in thirteen U.S. jurisdictions. In 2022, there were 121 measles cases and 49 in 2021.
Globally, measles cases increased by about 80% during 2022 compared with 2021.
For example, India has reported about 57,000 measles cases over the past year.
Measles is a highly contagious respiratory illness. The virus is transmitted by direct contact with infectious droplets or by airborne spread when an infected person breathes, coughs, or sneezes. According to the CDC, measles vaccination may prevent disease in exposed people within 72 hours of exposure.
If you think you have measles or have been exposed to someone with measles, isolate yourself from others and call your healthcare provider before arriving to be tested.
The incubation period averages 10-12 days from exposure to the onset of prodromal symptoms. People with confirmed or suspected measles should stay home from school, work, and other group settings until after the fourth day of rash onset.
The CDC says if you plan to travel internationally, ensure you and your loved ones are protected against measles before departure, no matter where you are going.
In the U.S., various measles vaccines are available at health clinics and pharmacies in September 2023.