Children located in a few African countries now have access to a second malaria vaccine, the R21/Matrix-M™ malaria vaccine.
As of December 21, 2023, the World Health Organization (WHO) has added this malaria vaccine to its list of prequalified vaccines, which increases access to vaccines procured by UNICEF and funding support for deployment by Gavi, the Vaccine Alliance.
In October 2023, WHO recommended that Oxford University's developed and Serum Institute of India manufactured R21/Matrix-M to prevent malaria in children following the advice of the WHO Strategic Advisory Group of Experts on Immunization and the Malaria Policy Advisory Group.
R21/Matrix-M integrates Novavax Inc.'s Matrix-M djuvant.
The initial malaria vaccine, Mosquirix™ RTS, S/AS01, obtained WHO prequalification status in July 2022.
"Today marks a huge stride in global health as we welcome the prequalification of R21/Matrix-M, the second malaria vaccine recommended for children in malaria-endemic areas, said Dr. Kate O'Brien, Director of WHO's Department of Immunization, Vaccines and Biologicals, in a press release on December 21, 2023.
"This is another step toward ensuring a healthier, more resilient future for those who have lived for too long in fear of what malaria could do to their children. Together with our partners, we are united in pursuing a malaria-free future, where every life is shielded from the threat of this disease."
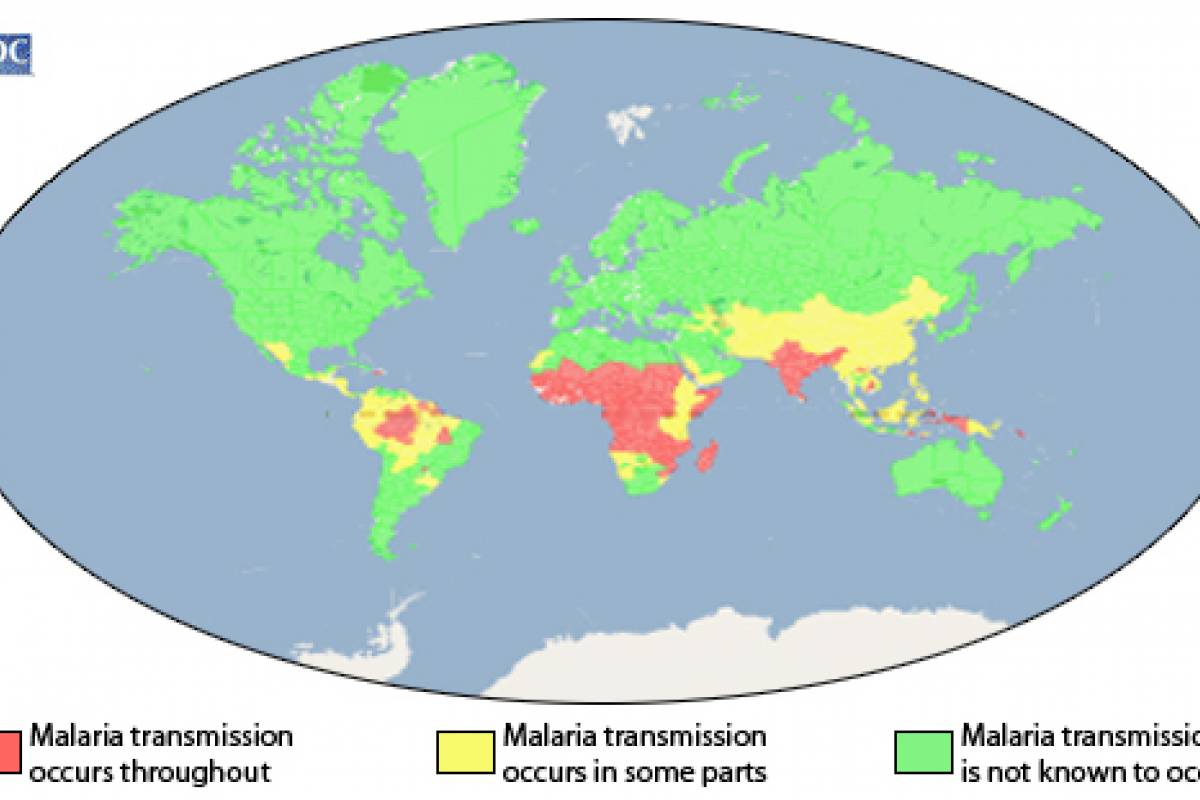
Malaria is a mosquito-borne disease causing a high burden on children in the African Region, where nearly half a million children die from the disease each year. Globally, in 2022, there were an estimated 249 million malaria cases and 608,000 malaria deaths across 85 countries.
In the United States, about 2,000 malaria cases are detected in international travelers annually. However, local malaria cases in the U.S. (Arkansas, Florida, Maryland, Texas) were detected in 2023.
These WHO-prequalified malaria vaccines are not available in the U.S.