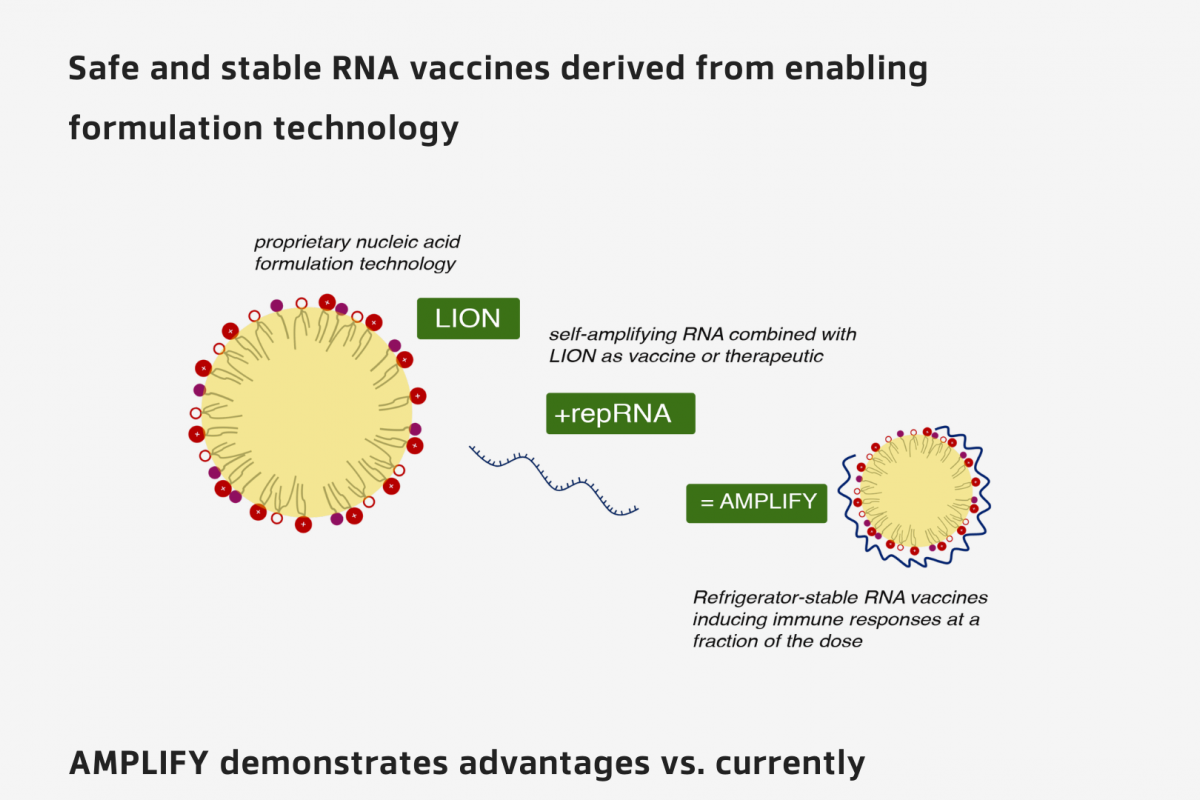
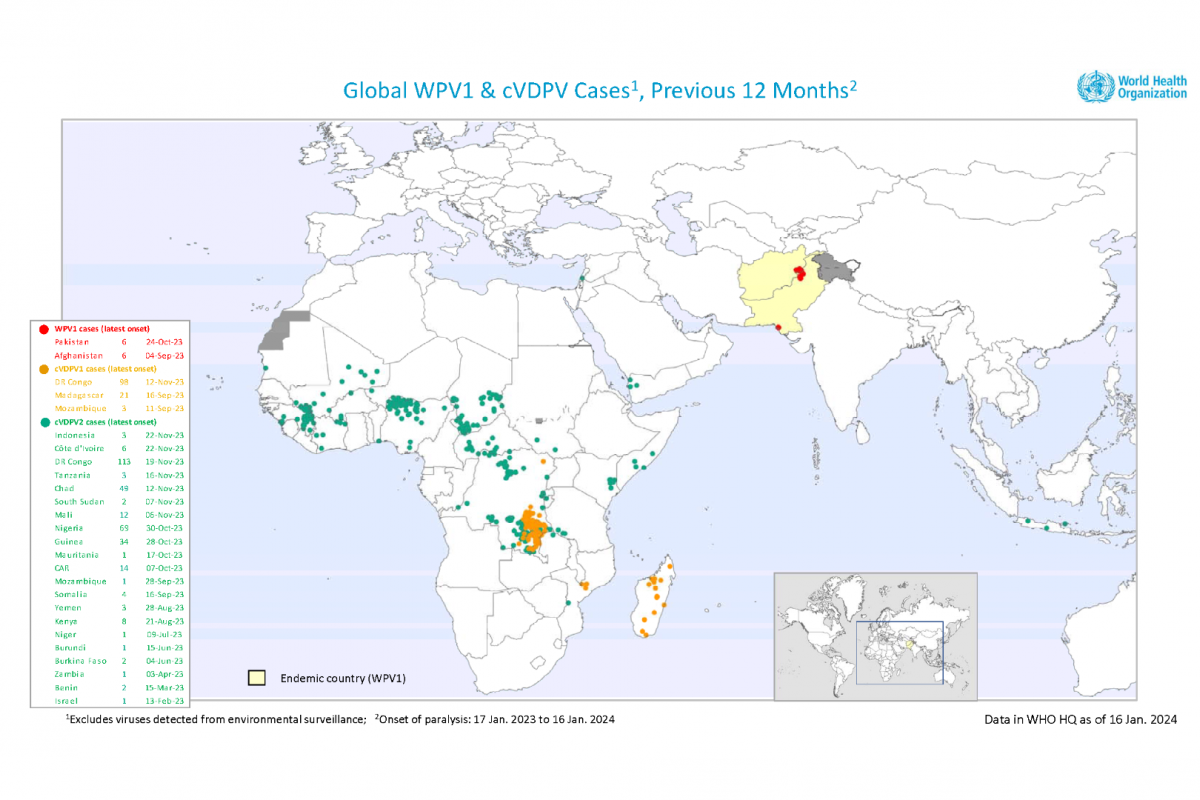
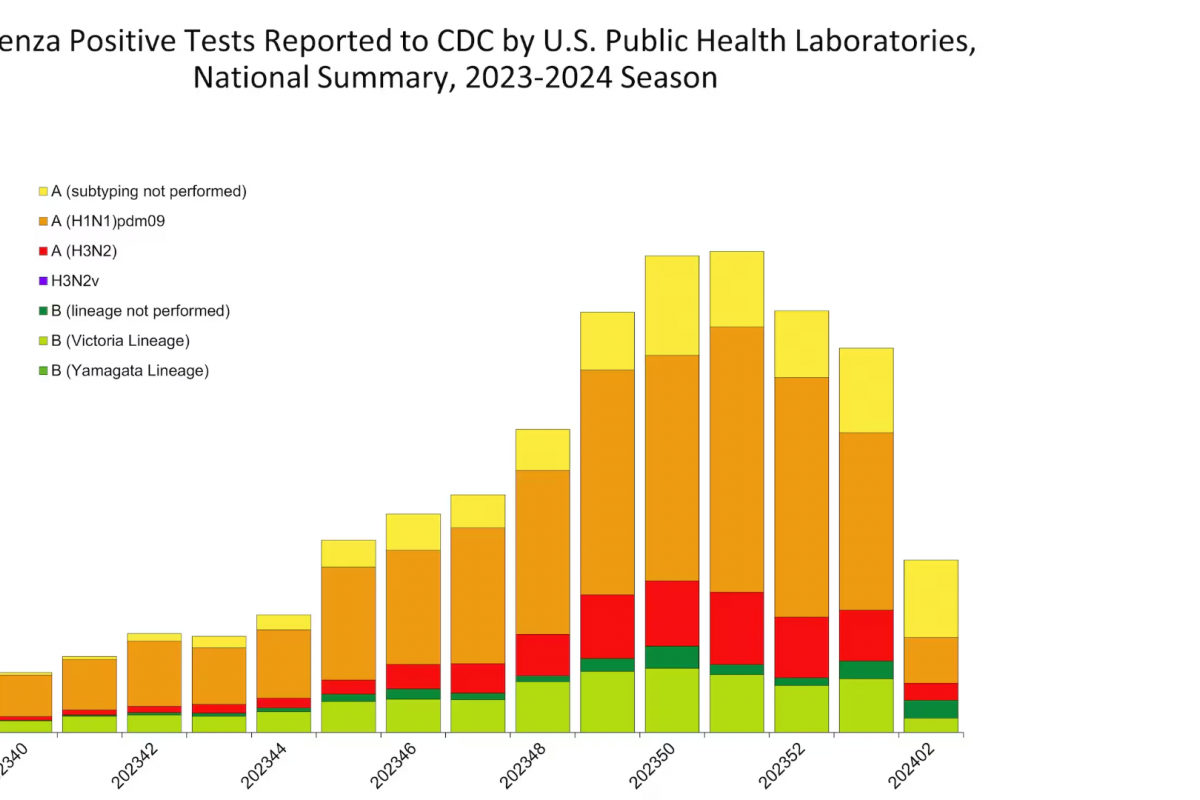
The WHO European Region confirmed today the measles virus spread in 41 of its 53 Member States in 2023. Among the countries most affected in the Region, the Republic of Kazakhstan has recorded the highest incidence, with 13,677 measles cases in 2023.
The majority of Kazakhstan measles cases were in children, 11,300.
As of January 23, 2024, there are 2,167 children in a Kazakhstan hospital with measles, 27 of them in a serious condition.
To implement measles vaccination campaigns, the Kazakhstan government purchased an additional 1.5 million doses of MMR vaccine in 2023.
"We have seen in the Region not only a 30-fold increase in measles cases ....This is concerning," explained Dr. Hans Henri P. Kluge, WHO Regional Director for Europe, in a press release in 2023.
"Vaccination is the only way to protect children from this potentially dangerous disease."
"Urgent vaccination efforts are needed to halt transmission and prevent further spread."
"It is vital that all countries are prepared to rapidly detect and respond promptly to measles outbreaks, which could endanger progress towards measles elimination," added Dr. Kluge.
In the United States, several cities, such as Atlanta, Kansas City, Philadelphia, and Wilmington, have reported measles cases in 2024.
The U.S. CDC says most measles cases in the U.S. are travel-related. In 2023, there were 56 measles cases reported by 20 U.S. jurisdictions.
Measles is a vaccine-preventable disease. Various measles vaccines are available at local pharmacies in the U.S.