The U.S. Centers for Disease Control and Prevention (CDC) published Key Updates for Week #2, ending January 14, 2023. This CDC report highlights both good and unfortunate news.
The Weekly U.S. Influenza Surveillance Report says seasonal influenza activity continues to decline across the U.S., with three regions below their outpatient respiratory illness baselines for the first time since October 2022.
And the majority of influenza viruses tested are in the same genetic subclade as and antigenically similar to the influenza viruses included in this season’s influenza vaccine, which remain available at most clinics and pharmacies in the U.S.
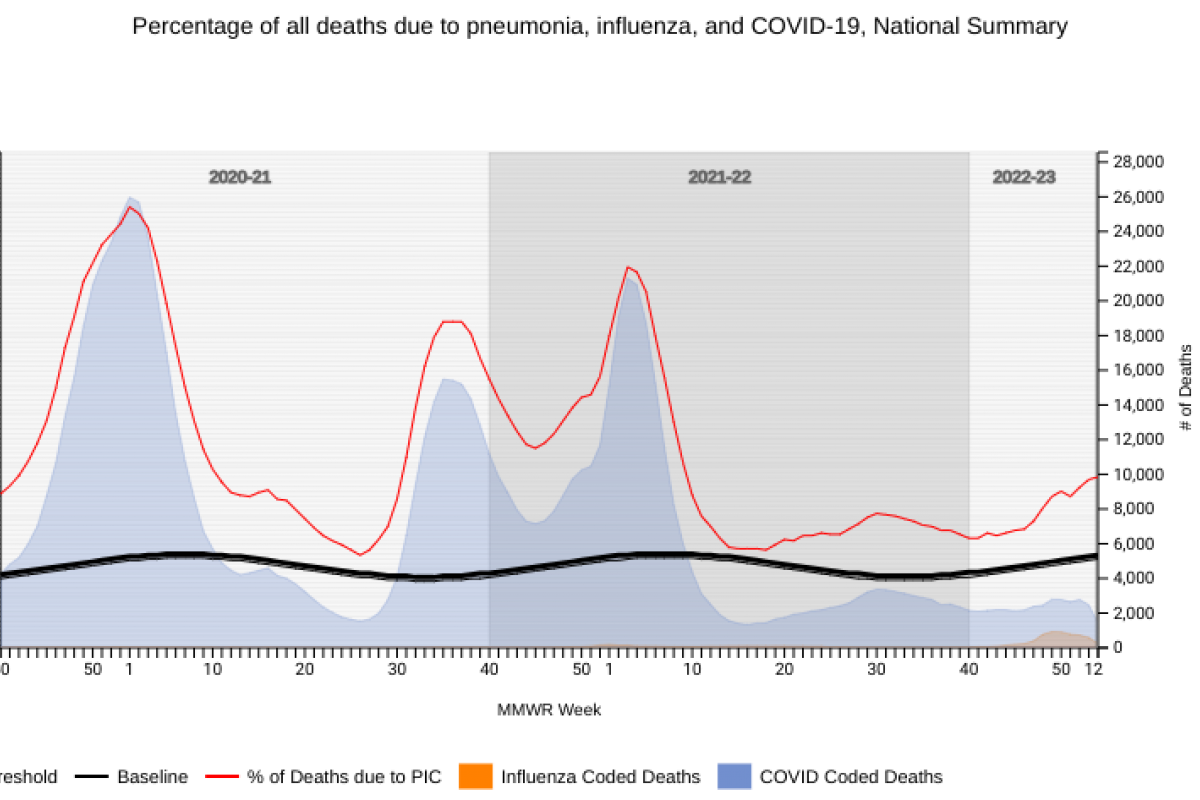
Furthermore, the National Center for Health Statistics Mortality Surveillance data available on January 19, 2023 shows that overall flu-related fatalities have decreased for the past four weeks.
During week #2, there were 2,954 pneumonia, influenza, and/or COVID-19 (PIC) deaths.
Among those PIC deaths, 1,422 had COVID-19 listed as an underlying or contributing cause of death on the death certificate, 1,281 documented pneumonia, and 251 listed influenza.
Unfortunately, the CDC also confirmed six additional influenza-associated pediatric fatalities have occurred during the 2022-23 flu season. This news increases the total of 85 pediatric flu deaths reported so far this season.
During the last flu season, there were only 45 pediatric fatalities related to the flu.
The CDC says an annual flu shot remains the best way to protect against influenza infections and can also prevent serious outcomes in people who get vaccinated but still get sick with the flu.
CDC recommends that everyone ages six months and older get an annual flu vaccine as long as flu activity continues, which could be several additional months.
So far this flu season, 171.52 million doses have been distributed in the U.S.