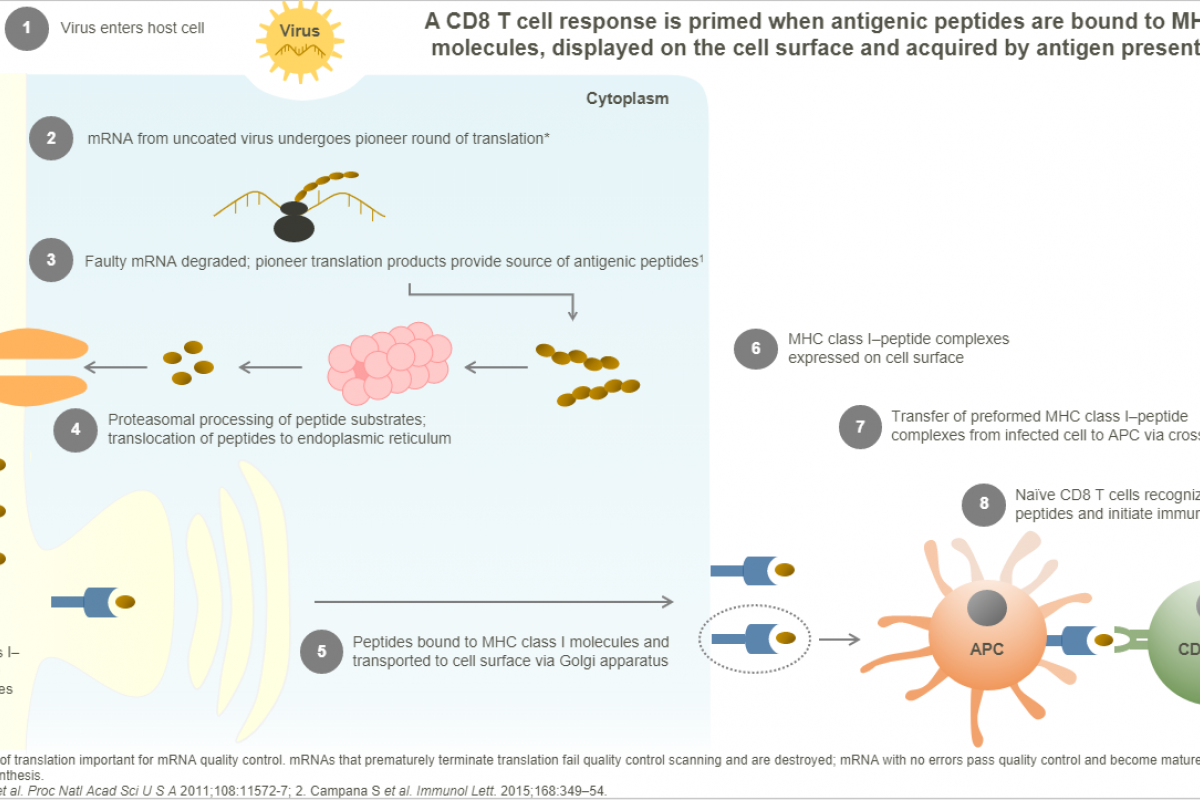
Emergex Vaccines Holding Limited today announced the successful completion of naNO-COVID, a Phase I clinical trial investigating the safety and reactogenicity of CoronaTcP™ in healthy volunteers.
CoronaTcP is Emergex's multi-target T cell-priming set-point product, designed to be broadly effective against diseases caused by betacoronaviruses, including SARS-CoV-1 and SARS-CoV-2 variants.
Cellular analyses demonstrated that CoronaTcP (two doses administered at Day 0 and 21) was able to activate virus-specific CD8+ T cells, with a significant increase in frequencies of CoronaTcP-specific CD8+ CD137+ CD69+ cells following in vitro antigenic stimulation in both low and high dose CoronaTcP groups at Day 35 post-treatment.
Significant changes were also observed for several virus-specific CD8+ memory subsets.
Professor Thomas Rademacher, Co-Founder and Chief Executive Officer of Emergex, said in a press release on July 19, 2023, Demonstrating that our platform has an acceptable safety profile and successfully mobilizes specific T cells that may elicit broad and long-term immune memory, validates our approach."
"By improving T cell-based immunity, we can enhance any previous immune status. We are delighted that this first assessment of treatment against infectious diseases for clinical use, based solely on a T cell response, was successful."
Overall, Phase I trial data validate Emergex's T cell-based approach to protection against RNA viruses and confirm the platform's potential using this innovative technology, supporting the investigation of other T cell-priming immune set-point candidates from the same platform.
In secondary immunogenicity analyses, several participants seroconverted during the trial (due to exposure to SARS-CoV-2) but had mild symptoms, confirming that CoronaTcP does not worsen an acute episode of COVID-19.
The naNO-COVID trial was a Phase I double-blind, randomized, base particle-controlled, single-center study designed to evaluate the safety and reactogenicity of two intradermal injections of an anti-Betacoronavirus candidate.