Search API
According to the World Health Organization (WHO), the seven cholera epidemic is considered to have started in 1961 and continues in March 2025.
So far, in 2025, a cumulative total of 70,488 cholera cases and 808 deaths were reported from 23 countries across three WHO regions.
In February 2025 alone, 459 cholera-related fatalities were confirmed, representing a 32% increase in January.
To help reduce cholera outbreaks, Oral Cholera Vaccine (OCV) production has remained high, reflecting significant efforts by the supplier and partners. The average OCV stockpile has recently stabilized at 5.5 million doses in February.
However, the WHO's external situation report #24 stated on March 20, 205, that the growing global demand continues to exceed supply, hindering efforts to control cholera outbreaks, respond rapidly to the disease’s spread, and implement preventative campaigns.
Cholera is an acute intestinal infection that spreads through food and water contaminated with the bacterium Vibrio cholerae, often from feces.
While cholera vaccination is not generally recommended for all international travelers, it is what the U.S. CDC suggested when visiting cholera outbreak zones.
In the United States, travel clinics and pharmacies currently have an ample supply of OCVs.

Since the Mpox virus swept around the world in May 2022, Germany's Standing Commission on Vaccination has recommended that people at an elevated risk of infection receive a preventive vaccination.
After millions of JYNNEOS® (MVA-BN®, IMVAMUNE®) doses were administered, an observational study published positive effectiveness data today.
The Lancet Infectious Diseases published results from a study conducted at Charité – Universitätsmedizin Berlin on March 18, 2025, that found one dose of the JYNNEOS was 84% in people without HIV and 58% effective against mpox infection overall.
However, due to the significant drop in Mpox infections in the second half of 2022, the study could not determine the additional effect of a second vaccine dose.
Furthermore, Breakthrough infections were associated with reduced symptoms, compared with infections in unvaccinated individuals.
In a related press release, Prof. Leif Erik Sander, Director of the Department of Infectious Diseases and Critical Care Medicine at Charité and a research group leader at the Berlin Institute of Health at Charité, stated, "Our results confirm that a single dose of the vaccine provides good protection against Mpox, at least for a short time."
"That is a very good figure, which is likely increased further by the second vaccine dose."
"The reason is that developing immune protection after vaccination presumably requires specific immune cells called T cells. These T cells often appear at lower levels in people with HIV and are not fully functional, which translates to a weaker immune response. This also corresponds to our observation that these participants experienced fewer local and systemic side effects after receiving the vaccine."
"We assume that people living with HIV develop protection against Mpox after the second vaccine dose, and urgently advise these people to receive the two vaccine doses."
"The immune system typically develops longer-lasting immune protection when exposed to the vaccine on more than one occasion."
Further studies will be required to determine the precise extent of the protective effect in different groups following two vaccine doses.
As of March 29, 2025, the JYNNEOS vaccine is commercially available at many clinics and pharmacies in the United States.

Since Queensland recorded a locally acquired human case of Japanese encephalitis (JE) in January 2025, residents have been urged to avoid being bitten by infected mosquitoes, which are being found along Australia's east coast.
This JE case, the first since 2022, may have infected the Darling Downs region, near Goondiwindi and Wide Bay regions, and animal populations in other jurisdictions.
On March 15, 2025, local health authorities confirmed the first Japanese Encephalitis Virus (JEV) detection in Hemmant, an eastern riverside suburb of Brisbane.
Brisbane is the capital and largest city of the state of Queensland, with a population of over 2.7 million.
According to a press release, Chief Health Officer Dr. Heidi Carroll said this latest detection meant JEV posed an increased risk of infection in humans across several regions in Queensland."
"This latest detection is the first for Brisbane and tells us that more mosquito populations are likely carrying the virus."
"While most people infected with JEV experience only mild or no symptoms, those who develop more serious symptoms may experience fever, headache, abdominal pain, or vomiting, typically within five to 15 days of being bitten."
"Tragically, in some cases, it can cause severe neurological illness and even death."
Furthermore, this year, JEV detections have been confirmed in New South Wales (NSW).
On March 14, 2025, the fourth JE-related fatality was reported since the virus was first detected in NSW in 2022.
In 2022, the U.S. CDC updated a Level 2 Practice Enhanced Precautions Advisory regarding the JE outbreak in eastern and southeastern Australia to alert international visitors to this health risk.
The CDC recommends JE vaccination for at-risk travelers before visiting outbreak areas.
The JEV vaccine is available at over 100 vaccination providers across Queensland. It is free for eligible Queenslanders. Since 2022, more than 18,000 Queenslanders have been vaccinated against JEV.

While the Federative Republic of Brazil continues to lead the Americas during the Chikungunya outbreak in 2025, with over 53,000 cases and 27 related fatalities, a small city recently confirmed an unusually high case fatality rate.
According to local media reporting on March 18, 2025, the city of Xanxerê registered its second Chikungunya-related fatality of 2025.
According to the Pan American Health Organization (PAHO), Chikungunya infections are seldom fatal. Estimates vary throughout the Americas, with case-fatality rates ranging between 0.5 and 1.3 deaths per 1000.
Located in Santa Catarina, southern Brazil, this city of just over 50 thousand inhabitants has recorded about 107 Chikungunya cases this year, indicating a very high fatality rate. This news article did not explain this data.
The PAHO says in Brazil, the mosquito that transmits Chikungunya to people is the same vector that transmits dengue fever and Zika virus, making Chikungunya easy to misdiagnose and appropriately treat.
From a disease prevention perspective, people have two options in March 2025.
The PAHO suggests avoiding being bitten by infected mosquitos.
And/or if you are departing abroad from the United States, the government suggests speaking with a travel vaccine expert about Chikungunya vaccination options before visiting an outbreak area.
The U.S. FDA has approved innovative Chikungunya vaccines for about two years. In 2025, they will be generally available at travel clinics and pharmacies.

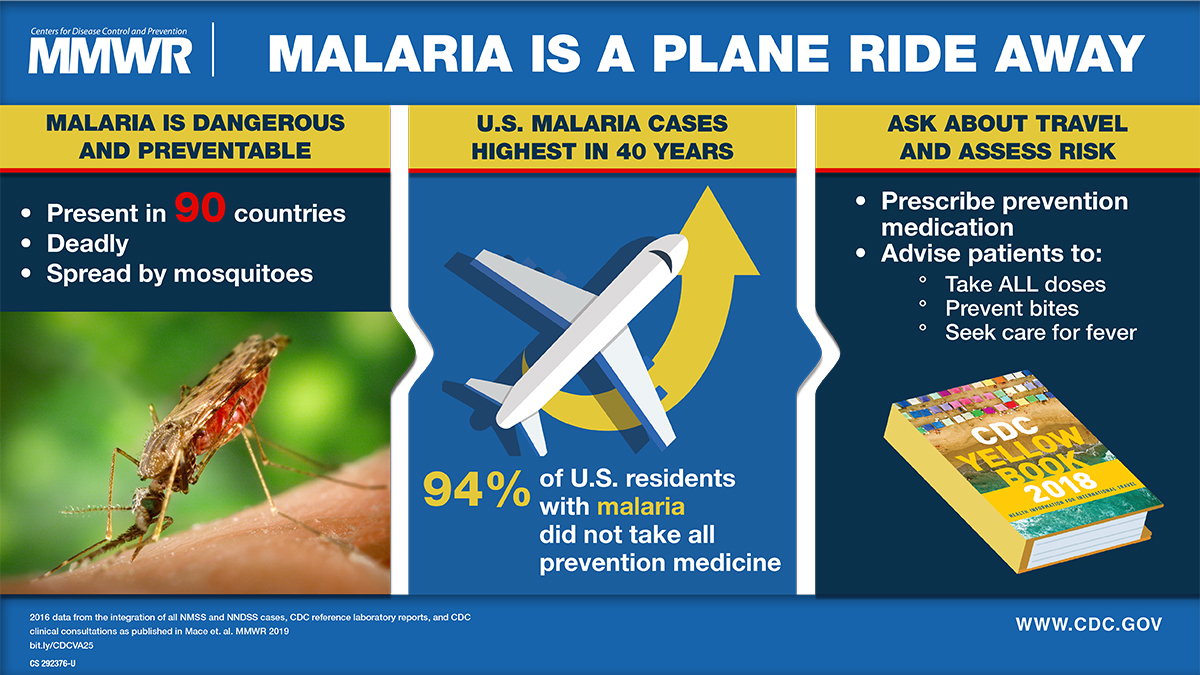
After a measurable decline in malaria cases globally over the last two decades, case numbers have rebounded throughout Africa during the previous two years, highlighting the need for enhanced prevention and treatment options.
According to the World Health Organization (WHO), despite the expenditure of $4 billion per year, malaria fatalities have not substantively been reduced during outbreaks.
While the WHO recommends two malaria vaccines (Mosquirix™ and R21 / Matrix-M™) to reduce mosquito-transmitted malaria outbreaks in Africa, a new study has identified a potential change in case management.
In The Lancet Infectious Diseases, Virak Eng and colleagues provide evidence of the benefit of high total-dose primaquine (7 mg/kg) compared with low total-dose primaquine (3·5 mg/kg) to prevent relapsing P vivax malaria in Cambodia.
These findings, published on March 17, 2025, and funded by the U.S. National Institutes of Health, provide strong evidence for the optimal primaquine dose for anti-relapse therapy and support the 2024 WHO malaria treatment guidelines update recommending high-dose primaquine in most endemic countries.
In the United States, most malaria cases are international travel-related, not locally transmitted.
Previously, the WHO estimated the annual global demand for malaria vaccines at 40–60 million doses by 2026. These vaccines are not commercially available in the U.S.
While a U.S. FDA-approved shingles vaccine has been well received in the market and numerous studies indicate it's effective and safe, a non-mRNA adjuvanted subunit vaccine candidate has completed a significant series B financing.
Announced on March 17, 2025, Curevo Vaccine closed a $110 million Series B round to advance the development of Amezosvatein, its vaccine for shingles (varicella-zoster virus).
"This Series B round will fund the extension of our successful Phase 2 program into an additional 640 participants, including the key population of adults over age 70, to finalize dose selection ahead of the Phase 3 program," said Curevo's CEO, George Simeon, MBA/MPH, in a press release.
"Designed based upon feedback from regulators and other stakeholders, this short extension trial will begin mid-2025 and set the company for clinical, strategic, and regulatory success."
The Phase 2 study (NCT05304351) 's primary completion date is March 31, 2025.
Like Shingrix®, amezosvatein, the assigned non-proprietary name for CRV-101, uses a subunit protein antigen called glycoprotein 'E' (gE). Targeting the gE antigen is proven to elicit a long‑term, protective immune response to prevent shingles.
Amezosvatein's adjuvant contains an optimized version of the TLR4 agonist proven by Shingrix to be biologically active in shingles vaccination.
Amezosvatein was engineered to maintain exceptional efficacy and have a best‑in‑class tolerability profile.
The SLA-SE adjuvant formulation was developed at Seattle‑based Access to Advanced Health Institute and amezosvatein was licensed from the Mogam Institute for Biomedical Research, a research institute funded by South Korea's GC Biopharma.
Until a phase 3 study is completed and approved by the FDA, this shingles vaccine candidate will not become commercially available in the U.S. Currently, the U.S. CDC recommends two doses of the recombinant zoster vaccine to prevent shingles and related complications in most people. This vaccine is offered at most pharmacies.
The Shingles Vaccine industry is projected to reach about $7 billion by 2032.