Search API
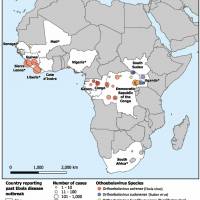
Since the Zika virus was first detected in the Americas in 2015, the Pan American Health Organization (PAHO) and the World Health Organization (WHO) have reported cases from 52 countries and territories in the region.
As of October 24, 2025, the PAHO Zika data dashboard indicates that there have been over 23,000 cases and four related fatalities in the Region of the Americas this year.
The current leaders are Brazil (21,762), Bolivia (1,012), and Argentina (849).
And in the Central America Region, Costa Rica, El Salvador, and Guatemala are the unfortunate leaders in reporting this mosquito-transmitted disease.
This PAHO data aligns with 2024, when 44,480 cases and two related fatalities were confirmed across all countries for the entire year.
Last year, Brazil reported about 90% of all cases.
In the Americas, one in four Zika-infected people may develop symptoms, but in those who are affected, the disease is usually mild, with symptoms lasting between 2 and 7 days. The clinical appearance is often similar to dengue or chikungunya.
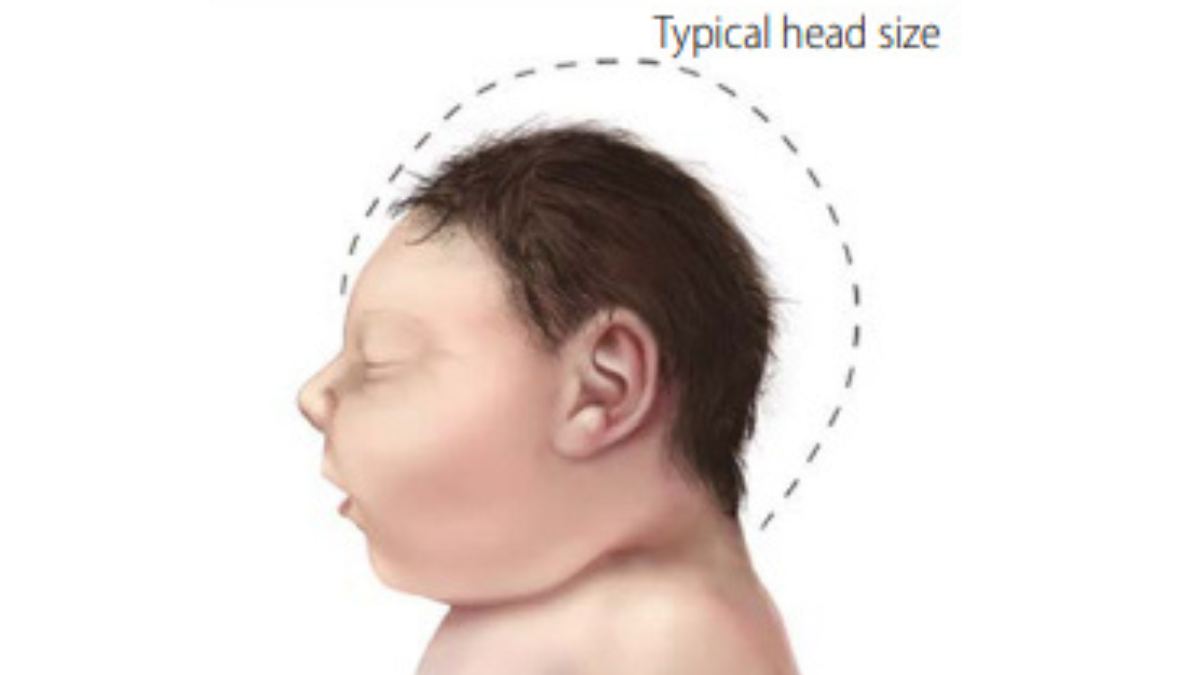
According to the PAHO, Zika virus infection during pregnancy poses severe risks to the fetus, ranging from congenital Zika syndrome to microcephaly to milder neurodevelopmental issues.
From a severity perspective, there is scientific consensus that Zika is a cause of Guillain-Barré syndrome.
As of October 2025, the UK travel advisory says that since there is no vaccination or medication to prevent Zika infection, women should avoid becoming pregnant while traveling to at-risk countries such as those listed above, and for 2 months after their last possible Zika virus exposure.
Prevention involves reducing mosquito populations and avoiding bites, which occur mainly during the day, says the UK advisory.
In the future, a Zika vaccine candidate may be approved for human use.
The European Center for Disease Prevention and Control (ECDC) has released updated information on the spread of West Nile virus (WNV) activity in Europe. Case numbers reported so far this year are above the average for the past decade.
During 2025, 13 European countries have confirmed 989 locally acquired cases and 63 related fatalities.
Locally acquired cases have been reported by Italy (714), Greece (91), Serbia (60), France (42), Romania (36), Spain (23), Hungary (11), Croatia (4), Albania (3), North Macedonia (2), Bulgaria (1), Kosovo* (1) and Türkiye (1).
The ECDC says Italy is currently experiencing a large outbreak, including 48 fatalities, with a case fatality rate of 6.7%.
The WNV cases have mainly been reported from the Lazio region (Latina, Roma, and Frosinone), with a total of 252 cases, and the Campania region (Napoli, Caserta, Salerno, and Avellino), with a total of 124 cases.
In the United States, WNV cases have been reported in various states, including Kentucky, in 2025.
Currently, WNV vaccines are in development and unavailable in Europe and the USA.
As of October 10, 2025, the U.S. CDC has not issued a Travel Health Notice regarding Europe's WNV outbreak.

Bavarian Nordic A/S recently reported topline results from a phase 2 clinical study of its MVA-BN® (JYNNEOS®) mpox/smallpox vaccine in children 2 to 11 years of age.
Topline results from the study, comprising 451 individuals evaluable for the primary endpoint, showed that the immune response in children (n=227) two weeks after the second vaccination with MVA-BN was non-inferior to the adult group (n=224), with the highest immune responses observed in the youngest subgroup of children aged 2-5 years.
While the safety and immunogenicity generated from this study in adults were comparable to historical data with MVA-BN, the immune response in children was 2.5 times higher than in the adult group, as demonstrated by neutralizing antibody titers.
The vaccine's safety profile is similar to that of adults and has no unexpected signals.
Nina Wressnigg, Head of Clinical Development Science at the Coalition for Epidemic Preparedness Innovations (CEPI), commented in a press release on October 7, 2025, "Mpox has been raging across Africa for over a year and remains a declared continental health emergency."
"Although MVA-BN has been licensed for emergency use in children in the Democratic Republic of the Congo (DRC) - the worst-affected country - many other countries lack this access, causing children to continue to bear the brunt of the suffering, marked by severe illness and possible loss of life.
"These new topline data provide additional positive findings that could expand licensure to children in more countries to control the ongoing outbreak."
Pending final results from the study, Bavarian Nordic plans to submit the data to the European Medicines Agency (EMA) in 2026 to support an extension of the vaccine's approval to include children aged 2 years and older.
The European Commission currently approves MVA-BN for individuals aged 12 years and older.
The findings could also expand the use of the vaccine to children in countries severely affected by the current mpox outbreak, surging in Africa, with cases also reported in other countries around the world.
MVA-BN or Modified Vaccinia Ankara-Bavarian Nordic is the only non-replicating mpox vaccine approved in the U.S., Switzerland, Singapore, Mexico, Canada (IMVAMUNE®), the EU/EAA, and the United Kingdom (IMVANEX®).
In the United States, JYNNEOS is commercailly available at clinics and pharmacies.
Recently, the Chicago Department of Public Health reported 104 cases of Mpox have been confirmed in 2025. This data is more cases than were reported over the same time period in 2023 (40) and 2024 (53) combined.
The study was co-funded by the CEPI and was conducted at sites in the DRC and Uganda.

PharmaJet today announced the expansion of its needle-free injection portfolio with the development of a suite of proprietary, needle-free, self-injector pens.
As of October 7, 2025, prototypes of the subcutaneous (SC) injectors, designed for the chronic disease and metabolic peptide self-injection markets, are currently undergoing early testing.
PharmaJet's needle-free injector pens aim to enhance the user experience, alleviate administration anxiety and discomfort, and simplify the injection procedure, particularly for patients with mobility challenges.
Importantly, the reusable PharmaJet injector pens may offer significant advantages over current disposable technologies that generate enormous waste.
"We are excited about the prospect of entering the pen injector market with the PharmaJet needle-free self-injector pens," said Wouter Latour, President and CEO of PharmaJet, in a press release.
"The PharmaJet devices are being designed to provide a superior, user-experience to a wide group of patients, while offering smart e-technology to track dosing and support patient compliance."
"We plan to reach out to industry in the coming months to build partnerships in the exponentially growing metabolic peptide segment and other markets where frequent injections are important."
PharmaJet is a commercial-stage world leader in needle-free injection systems. More than 12 million vaccine injections have been administered worldwide using Tropis®, PharmaJet's intradermal Needle-free Injection System.
Published research comparing Tropis with needle and syringe shows that it is preferred by over 95% of caregivers, and recipients reported reduced pain (68%) and soreness (69%).
PharmaJet's Needle-free Injection Systems deliver a spring-powered injection in 1/10 of a second by means of a narrow stream of fluid that penetrates the skin with a precise dose and depth.
The Stratis System has received U.S. FDA 510(k) marketing clearance, a CE Mark, and WHO PQS certification, enabling the delivery of medications and vaccines via intramuscular or subcutaneous routes. Tropis® ID has CE Mark and WHO PQS certification for intradermal injections and is commercially available for global immunization programs.