Search API
After a record-setting 2024, São Paulo state in Brazil is on pace to report a significant Dengue virus outbreak in 2025.
In the first month of 2025, São Paulo's Dengue dashboard shows over 108,000 cases and 25 related fatalities.
The state of São Paulo is a tourist hot-spot, welcoming over 6 million visitors last year.
Nationally, the Pan American Helath Organization reported Brazil had confirmed about 194,000 probable Dengue cases in January 2025.
The U.S. CDC says that Dengue is a year-round risk in various countries in the Region of the Americas. And the agency has identified a higher-than-expected number of Dengue cases among U.S. travelers returning from those countries.
To alert international travelers to this health risk, the CDC republished a Level 1 Travel Health Advisory in 2024, identifying 12 countries in the Americas reporting Dengue outbreaks. The CDC strongly recommends avoiding mosquito bites when visiting Dengue-endemic areas in Brazil.
While other countries have approved a second-generation Dengue vaccine in 2025, the U.S. has not.
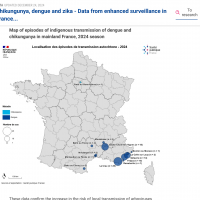
The Pan American Health Organization (PAHO) has confirmed local transmission of the Zika virus in countries and territories in the Region of the Americas for several years.
As of February 1, 2025, 303 Zika cases were reported in the Americas this year, with cases reported in Brazil, Bolivia, and Colombia.
Last year, the PAHO reported a 13% increase in cases.
Over 42,127 ZIka cases and two related fatalities in the Americas in 2024.
In 2024, very few cases were reported in the United States. The CDC's preliminary data show 19 travel-related cases and nine locally acquired cases in U.S. territories, such as Puerto Rico.
While the CDC does not recommend any Zika vaccine candidate, Valneva SE's VLA1601 second-generation purified, inactivated, whole Zika virus vaccine candidate is the most advanced Zika vaccine in development. However, this innovative vaccine will probably not be commercially available in 2025.
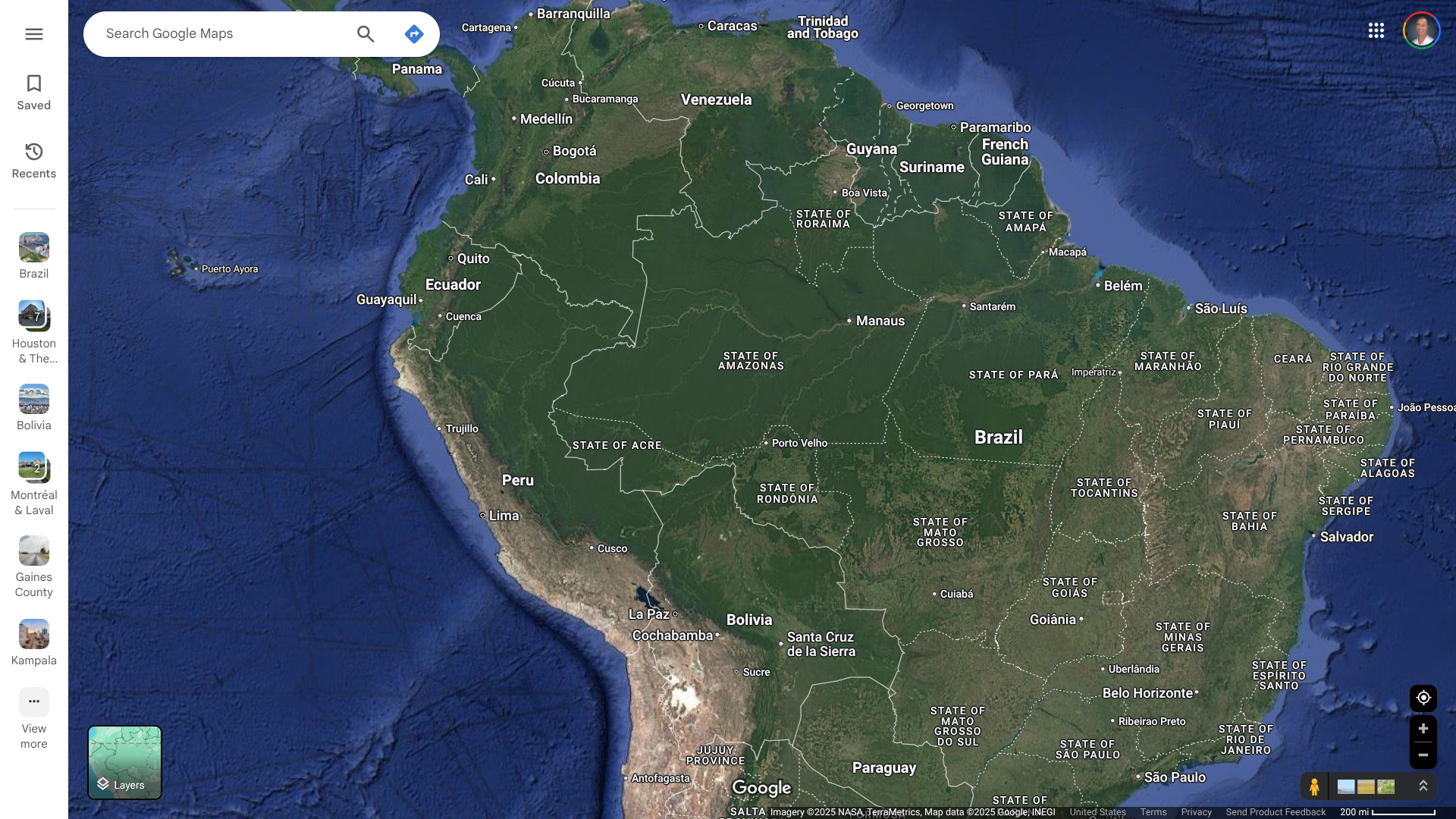
During the summer of 2024, the Pan American Health Organization (PAHO) issued several alerts that revealed a spike in Oropouche disease cases, including fatalities, in the Region of the Americas.
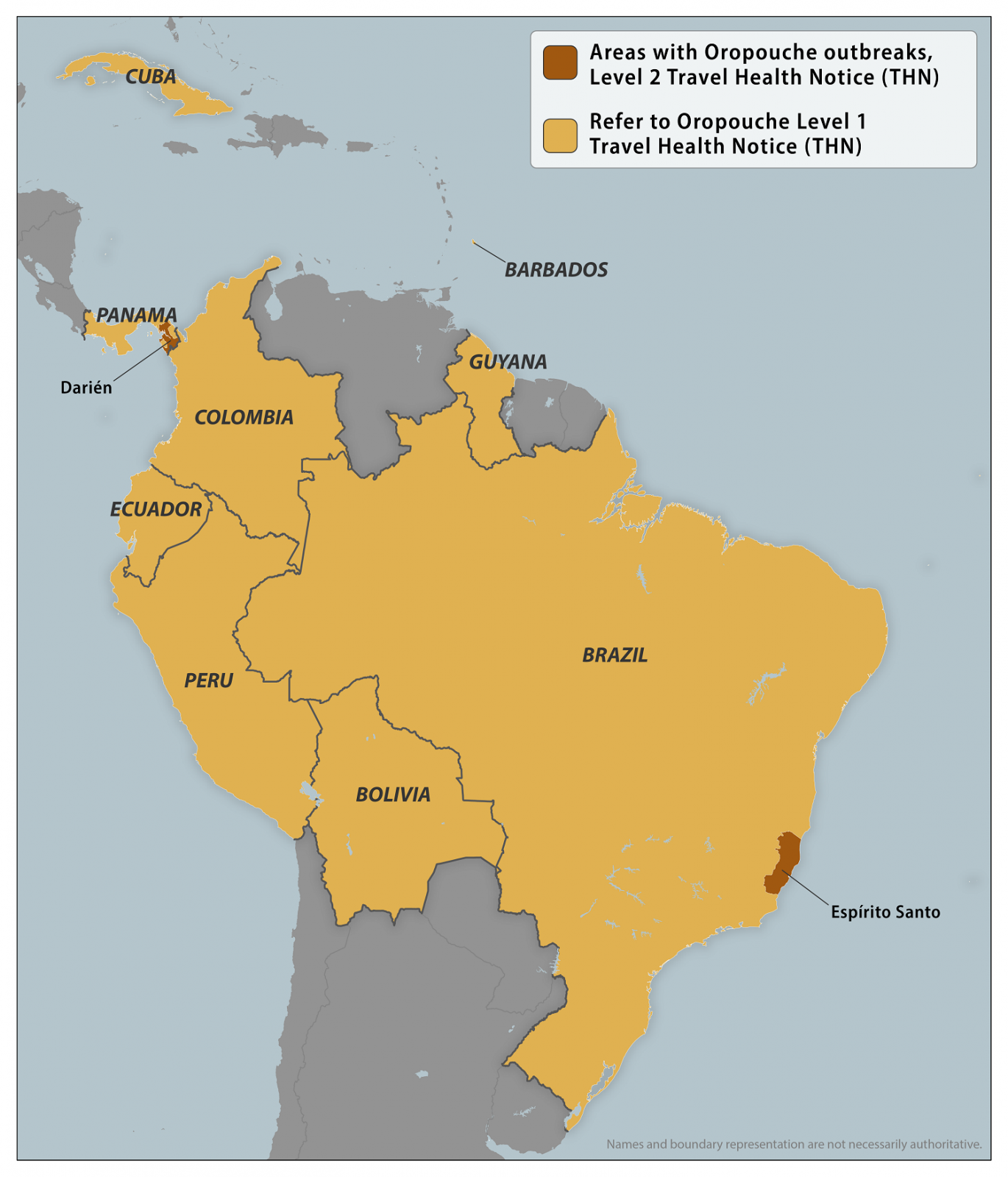
As of January 31, 2025, the U.S. CDC confirmed recent outbreaks of Oropouche in Espírito Santo, Brazil, and Darién Province, Panama. To notify travelers of this health risk, the CDC issued a Level 2 Travel Health Notice.
It says all travelers to these areas should prevent bug bites during travel to protect themselves from infection. They should also prevent bug bites for 3 weeks after travel to avoid possibly spreading the virus to others if they are in areas where mosquitoes and biting midges are active.
Furthermore, healthcare providers should inform women who are pregnant and considering travel to areas with reported Oropouche virus transmission of the possible risks to the fetus. If a pregnant woman decides to travel, counsel her to prevent bug bites.
This new Travel Health Notice follows a Level 1 alert issued in 2024.
The CDC says there is no evidence of Oropouche virus transmission in the United States, but various states (Florida) have reported cases detected in international travelers.
In 2024, 108 neuroinvasive and non-neuroinvasive Oropouche cases were reported in six U.S. jurisdictions. The CDC also says that the extent to which the Oropouche virus could spread in Puerto Rico and the U.S. Virgin Islands is unknown.
From a prevention perspective, since no approved vaccines are available in February 2025, avoiding bug bites is a person's best option.
While Japanese encephalitis virus (JEV) cases are rare in the United States, this mosquito-transmitted virus is the leading cause of viral encephalitis in many countries of Asia and Pacific countries, with an estimated 100,000 clinical cases every year.
To ensure its military personnel are fully protected from JEV infection, the U.S. Department of Defense (DoD) initiated a new $32.8 million contract with Valneva SE to supply its Japanese encephalitis (JE) vaccine, IXIARO®. The new contract will commence immediately.
Under this one-year contract, the DoD can purchase additional doses during twelve months.
Dipal Patel, Chief Commercial Officer of Valneva, commented in a press release on January 30, 2025, “We are honored to continue our long-term relationship with the DoD. The U.S. military has trusted IXIARO® for over ten years to help protect military personnel, their families, civilian government service personnel, and government contractors from this potentially deadly disease.”
Deliveries of IXIARO® doses have continued in 2024 under the DoD supply contract signed in September 2023.
According to the World Health Organization, JE is fatal in approximately 30% of those who show symptoms and leaves half of survivors with permanent brain damage. The disease is endemic in Southeast Asia, India, and China, regions with more than three billion populations.
In 2024, JEV was detected in mosquitoes in various areas in Australia.
NSW Health Dr. McAnulty commented in a related press release, “These detections indicate the risk for mosquito-borne virus transmission is widespread, particularly in the NSW local government areas at higher risk of JE in the inland regions."
“I encourage anyone planning to spend time outdoors in these higher-risk areas to take steps to protect against mosquito bites."
Since millions of international travelers visit Australia annually, the U.S. CDC suggests travelers speak with a travel vaccine expert about immunization options before traveling abroad. In the U.S., IXIARO® is commercially offered at travel clinics and pharmacies.

Merck announced today that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) recommended the approval of CAPVAXIVE™ (Pneumococcal 21-valent Conjugate Vaccine) for active immunization for the prevention of invasive disease and pneumonia caused by Streptococcus pneumoniae in individuals 18 years of age and older.
Pneumococcal disease is an infection caused by Streptococcus pneumoniae. There are about 100 types of pneumococcal bacteria, and they can affect adults differently than children.
“Invasive pneumococcal disease and pneumococcal pneumonia remain critical public health challenges worldwide,” said Dr. Paula Annunziato, senior vice president of infectious diseases and vaccines, Global Clinical Development, Merck Research Laboratories, in a press release on January 31, 2025.
The CHMP’s recommendation for marketing authorization in the European Union (EU), Iceland, Liechtenstein, and Norway will now be reviewed by the European Commission. A final decision is expected by the second quarter of 2025.
If approved in the EU, it would mark the fourth authorization of CAPVAXIVE for preventing invasive pneumococcal disease and pneumococcal pneumonia in adults.
CAPVAXIVE was first approved in the U.S. in June 2024, Canada in July 2024, and Australia in January 2025. It is being reviewed in Japan, and other worldwide regulatory filings are underway.
In the U.S., pneumococcal vaccines are recommended for most people and are available at most community pharmacies. These vaccines may not work for everyone.
During 2024, the United States reported several measles outbreaks primarily related to unvaccinated international travelers. According to new reports, the State of Texas may lead this unfortunate list in 2025.
Today, the Texas Department of State Health Services (DSHS) announced two confirmed measles cases in Gaines County residents, located southwest of Lubbock, Texas. Both instances involve unvaccinated school-age children who were hospitalized in Lubbock.
As of January 30, 2025, these children have been discharged.
These newly identified cases are in addition to two confirmed measles cases reported in Harris County in 2025.
The Houston Health Department (HHD) identified two confirmed measles cases associated with international travel. Both adults were unvaccinated against measles.
HDD says anyone exposed to measles should monitor themselves for symptoms, including a rash, high fever, cough, runny nose, and red, watery eyes. Symptoms can appear 7 to 21 days after exposure. If you show symptoms of measles, call your healthcare provider to make arrangements for evaluation and treatment.
On January 23, 2025, HHD stated, 'Due to the highly contagious nature of this disease, additional (measles) cases may occur.'
Houston and Harris County are home to about 5 million people and are gateway cities with two international airports.
Crockett Tidwell RPh, CDCES, CTH, informed Vax-Before-Travel News, "Measles is extremely contagious; nine9 out 10 people in the same room will become infected if they do not have immunity."
"All it takes is one international traveler to infect every vulnerable person they come in contact with when they come home," added Tidwell, Clinical Services Manager, International Society of Travel Medicine Certificate in Travel Health™.
To alert travelers of the global measles risk, the U.S. CDC recently updated a Travel Health Advisory, which identified 59 countries reported measles cases. The CDC recommends people speak with a travel vaccine expert about immunization options before visiting these countries in 2025.

Takeda today announced earnings results for the third quarter of fiscal year 2024 (nine months ending December 31, 2024), showing continued advancement and demand for its dengue virus vaccine, Qdenga®.
The Company reported Qdenga's FY2024 H1 revenue was JPY 19.9B, reflecting 863% growth.
As of January 30, 2025, Qdenga is available in 27 countries, including 19 European countries, with travel recommendations to support using Qdenga to help protect travelers to dengue endemic areas.
For example, over the past year, dengue outbreaks have set new records in countries throughout the Region of the Americas.
In 2024, cities in the United States reported local dengue infections, including Los Angeles, California, and Miami, Florida.
Previously, the World Health Organization added Qdenga to its List of Prequalified Vaccines, which should expand the number of countries offering this second-generation dengue vaccine.
Unfortunately, Qdenga is not available in the United States.
On July 11, 2023, Takeda voluntarily withdrew the Biologics License Application following discussions with the U.S. Food and Drug Administration. As of 2025, there has been no indication approval discussions were pending.
Note: This VBT news article was update don January 5, 2025, to include current country authorizations.

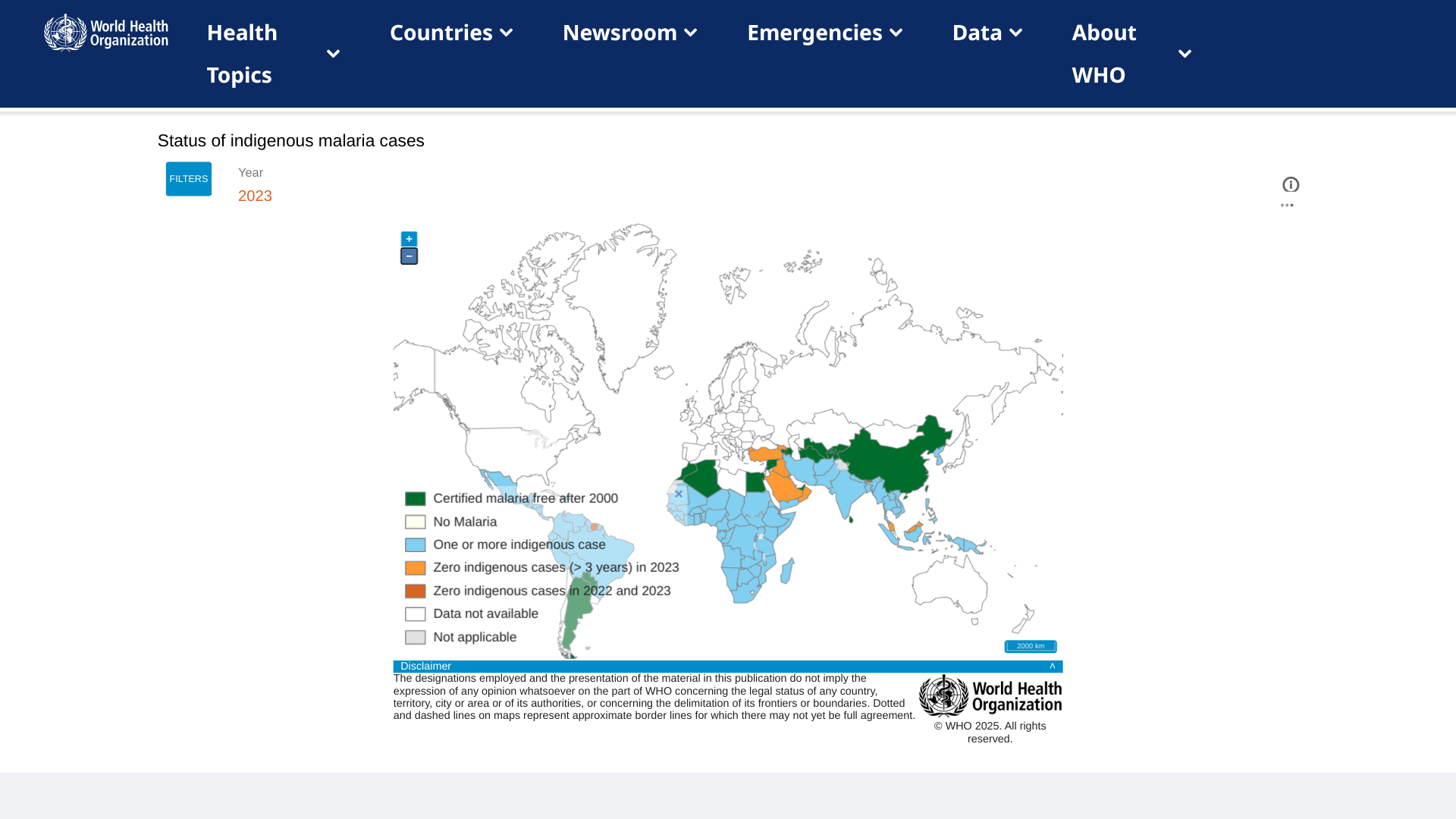
Ocean Biomedical recently announced that its Scientific Co-founder, Dr. Jonathan Kurtis, MD, PhD, and his research team have received additional funding from the U.S. National Institutes of Health (NIH) to advance their malaria vaccine research.
With the support of a $4.6 million non-governmental Foundation grant, Dr. Kurtis’ team is now testing three vaccine candidates in non-human primates. These candidates aim to block the malaria parasite’s ability to enter and exit red blood cells.
The research also explores the feasibility of using lipid-encapsulated messenger ribonucleic acid (mRNA) technology as a delivery mechanism.
In December 2024, Dr. Kurtis secured a $3.5 million NIH grant to identify vaccine targets further to protect against severe malaria in children.
Malaria remains a devastating global health challenge, claiming the lives of over 500,000 children annually in sub-Saharan Africa.
As of January 30, 2025, two malaria vaccines are available in Africa. However, they are not available in the U.S.