Search API
The World Health Organization (WHO) recently published a technical brief on encephalitis, a serious, life-threatening neurological condition characterized by brain inflammation.
On February 17, 2025, the WHO stated that different pathogens, such as herpes simplex virus (HSV), can cause encephalitis.
Some pathogens, like the Japanese encephalitis virus (JEV), are spread by mosquitoes and ticks, but vaccination can prevent transmission.
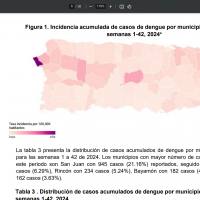
As of 2025, JEV outbreaks are the leading cause of viral encephalitis in twenty-four countries in the WHO South-East Asia and Western Pacific Regions, exposing more than 3 billion people to infection risks.
For example, the WHO reported various JEV cases across Australia, including Queensland, New South Wales, South Australia, Victoria, the Australian Capital Territory, Tasmania, Western Australia, and the Northern Territory.
Encephalitis affects people across all age groups, has high mortality, and often leads to significant long-term complications (sequelae), including hearing loss, seizures, limb weakness, and difficulties with vision, speech, language, memory, and communication.
Globally, in 2021, encephalitis was the fourth leading cause of neurological health loss in children aged under 5 years and the 13th across all age groups.
"Encephalitis is a growing public health challenge, and by prioritizing it within global and national health agendas and strengthening collaboration, we can reduce its impact and save lives," said Dr Tarun Dua, Head of the Brain Health Unit, WHO, in a press statement.
The WHO technical brief, which forms part of the implementation of the broader Intersectoral global action plan on epilepsy and other neurological disorders, draws attention to the lack of access to essential care, especially in low-and middle-income countries.
While no HSV vaccines are authorized, the U.S. FDA-approved JEV vaccine, IXIARO®, is available at clinics and pharmacies nationwide. According to the U.S. CDC, vaccination is recommended before visiting JEV outbreaks.

After almost two years without reporting a measles case, the Texas Department of State Health Services (DSHS) today confirmed 92 measles cases in this year's outbreak.
Since the beginning of 2025, there have been two measles cases in Harris, 57 in Gaines, 20 in Terry, 6 in Dawson, 4 in Yokaum, 1 in Ector, and 1 in Lynn Counties.
Most of these patients are young, unvaccinated, or their vaccination status is unknown.
On February 21, 2025, the DSHS stated, 'Due to the highly contagious nature of this disease, additional cases are likely to occur in the outbreak area and the surrounding communities. DSHS is working with local health departments to investigate the outbreak.'
Nationwide, the U.S. CDC updated its reporting yesterday but only indicated 93 measles cases in 8 jurisdictions: Alaska, California, Georgia, New Jersey, New Mexico (9), New York City, Rhode Island, and Texas.
DSHS and the CDC strongly recommend most people get the MMR vaccine to prevent infection and severe disease.

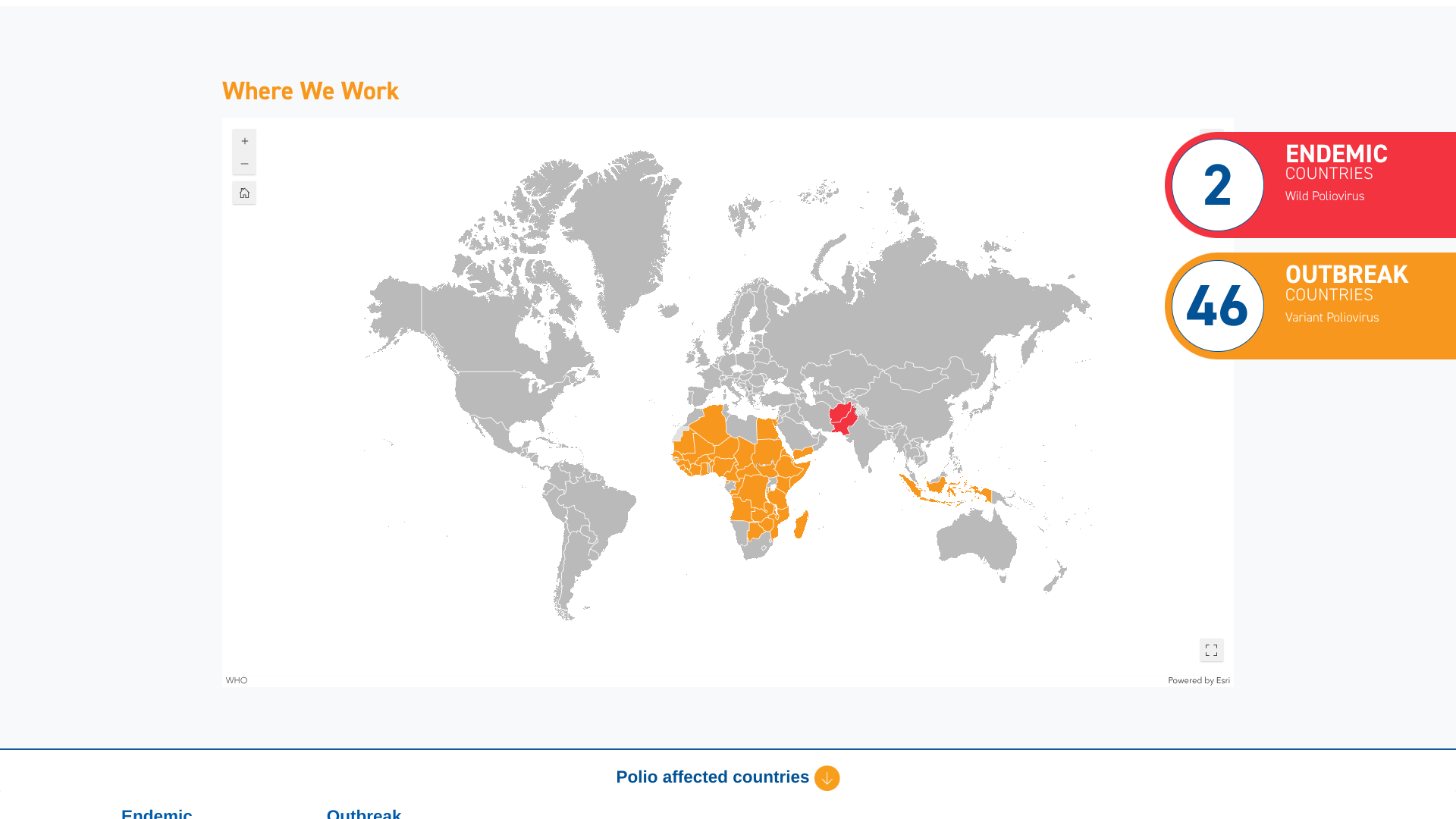
Health ministries worldwide recently met at the WHO Executive Board meeting in Geneva. They expressed serious concern about the increasing transmission of wild poliovirus in Asia's last two endemic countries: Pakistan and Afghanistan.
The total number of polio cases in Afghanistan for 2024 was 25, and Pakistan reported 74 cases in 2024.
These ministers noted that 'extraordinary measures were being taken in response.'
WHO Member States commended the implementation of new emergency operational approaches in both countries.
These approaches include identifying the different operational reasons why children are not being reached, area by area; increasing co-administration of inactivated polio vaccine (IPV) alongside oral polio vaccine (OPV); boosting overall immunity levels in children; and improving access to more adequate sanitation infrastructures.
Member States expressed concern about the ongoing variant poliovirus outbreaks (circulating vaccine-derived polioviruses) and urged intensified response to stop these outbreaks by maximizing the impact of novel oral polio vaccine type 2 (nOPV2).
This triple-locked polio vaccine has been administered over 1 billion times recently.
Speaking on behalf of the Eastern Mediterranean Region, Regional Director Dr Hanan Balkhy commented in a media statement, “I assure you, on behalf of our Region and the leaders of both endemic countries, our commitment to eradicating this virus is stronger than ever. We must reach and vaccinate every child and keep up a robust search for poliovirus, to stop further spread."
In Europe, the unusually high number of poliovirus detections has reinforced the urgency of the wake-up call. From September through December 2024, vaccine-derived poliovirus type 2 was detected in wastewater systems of 14 cities in five European countries.
As of February 21, 2025, IPV vaccination is recommended for most people in the United States. Furthermore, a booster dose may be advised for international travelers visiting polio outbreak areas.
Dynavax Technologies Corporation today confirmed it continues developing a plague (rF1V) vaccine candidate adjuvanted with CpG 1018® in collaboration with, and fully funded by, the U.S. Department of Defense (DoD).
As of February 20, 2025, based on the results from a randomized, active-controlled Phase 2 clinical trial, Dynavax and the DoD executed a new agreement for approximately $30 million through the first half of 2027 to support additional clinical and manufacturing activities, including a Phase 2 clinical trial expected to initiate in the third quarter of 2025.
As previously announced, Dynavax and the DOD executed an earlier agreement providing approximately $22 million in funding to develop the rF1V vaccine.
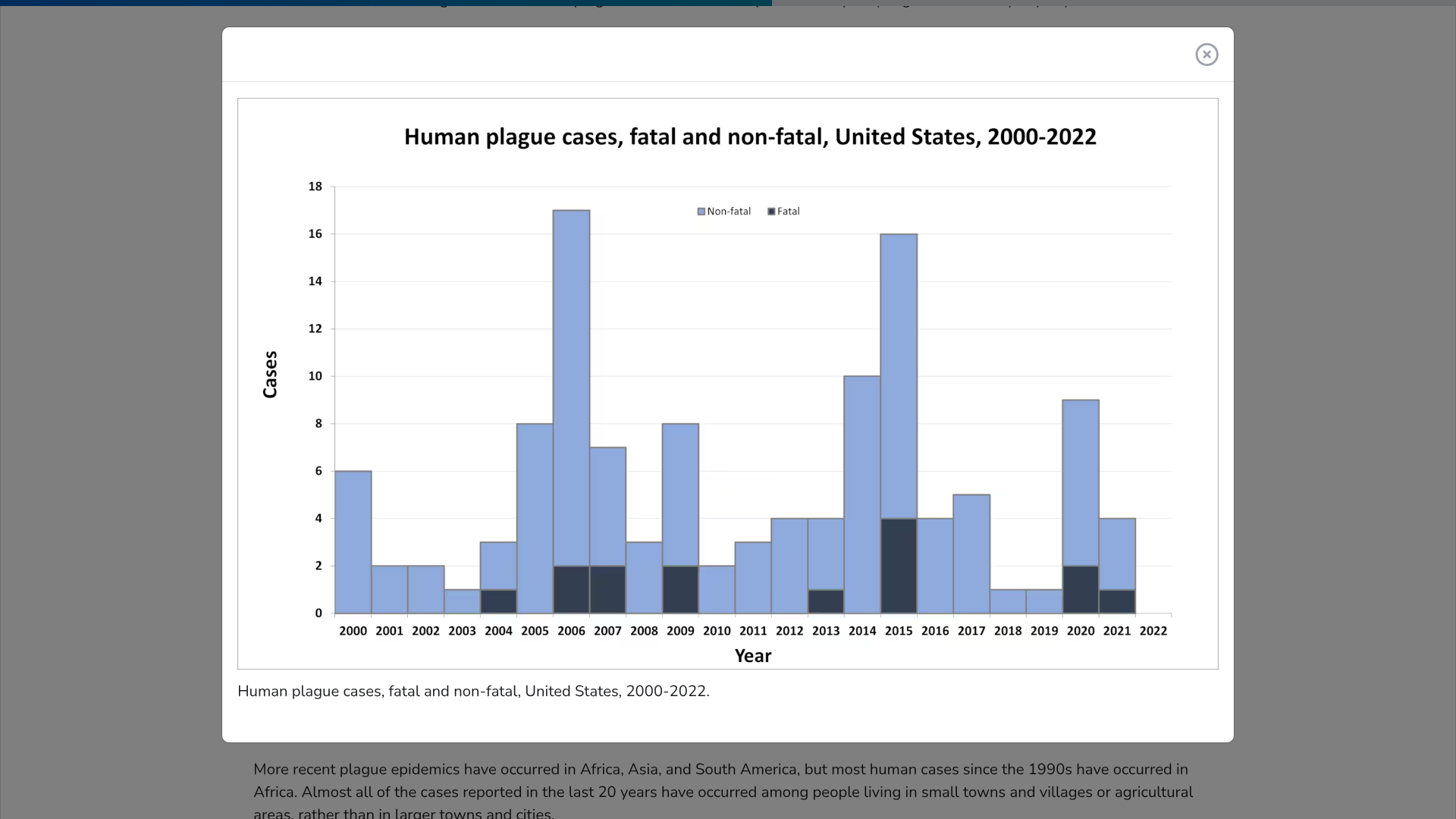
According to the U.S. CDC, Plague is a potentially deadly infectious disease caused by bacteria found in fleas and rodents or by handling an infected animal. It is caused by the bacterium Yersinia pestis. It is possible that Pneumonic plague bacteria could be released intentionally in a biological attack to sicken people.
Since the mid–20th century, plagues in the United States have typically occurred in the rural West. The CDC says cases in the eastern United States are among people who traveled from the west or have laboratory exposure.
More recent plague epidemics have occurred in Africa, Asia, and South America.
Throughout the record-setting Dengue fever outbreak in the Region of the Americas in 2024, most international travelers were unprotected when visiting at-risk countries such as Brazil, Costa Rica, and even Miami Breach.
Furthermore, once infected with this mosquito-transmitted viral disease, patients could not access innovative therapies.
To accelerate the approval of Dengue treatments, the U.S. National Institutes of Health (NIH) is testing an experimental treatment.
The phase 2 clinical study will involve exposing volunteers to a weakened strain of dengue virus that causes a mild form of the disease and administering an investigational therapeutic at various doses to assess its safety and ability to lessen symptoms.
This NIH clinical trial will test the ability of AV-1, an investigational human monoclonal antibody (mAb) therapeutic developed by AbViro, to mitigate clinical symptoms when administered before and after dengue virus infection at Johns Hopkins Bloomberg School of Public Health Center for Immunization Research and the University of Vermont.
However, none of the volunteers will develop dengue fever or severe dengue during this study.
“When caring for a patient who is critically ill with dengue, healthcare providers have few options other than providing supportive care,” said NIAID Director Jeanne Marrazzo, M.D., M.P.H., in a press release on February 11, 2025.
“We must find safe and effective therapeutics to provide much-needed relief to people suffering from dengue.”
The researchers will use this information to determine how AV-1 affects the volunteers’ ability to recover from dengue compared to placebo and to determine the dosages at which AV-1 may be effective.
If AV-1 shows promising results in this clinical trial, researchers may pursue further clinical evaluations of its safety and efficacy against the dengue virus.
The results of a previously completed Phase 1 trial indicated that AV-1 is safe in humans, providing the basis for the new clinical trial to test its safety and efficacy.
Another Dengue mAb candidate, Dengushield (VIS513), is being evaluated in phase 2 clinical trials.
This mAb is a highly potent inhibitor of all four types of dengue viruses, both in vitro and in preclinical animal models. Dengushield was licensed to the Serum Institute of India Pvt. Ltd. for development.
Despite these potential innovations, during Spring Break 2025, preventing mosquito bites is the best tactic to avoid Dengue infection.

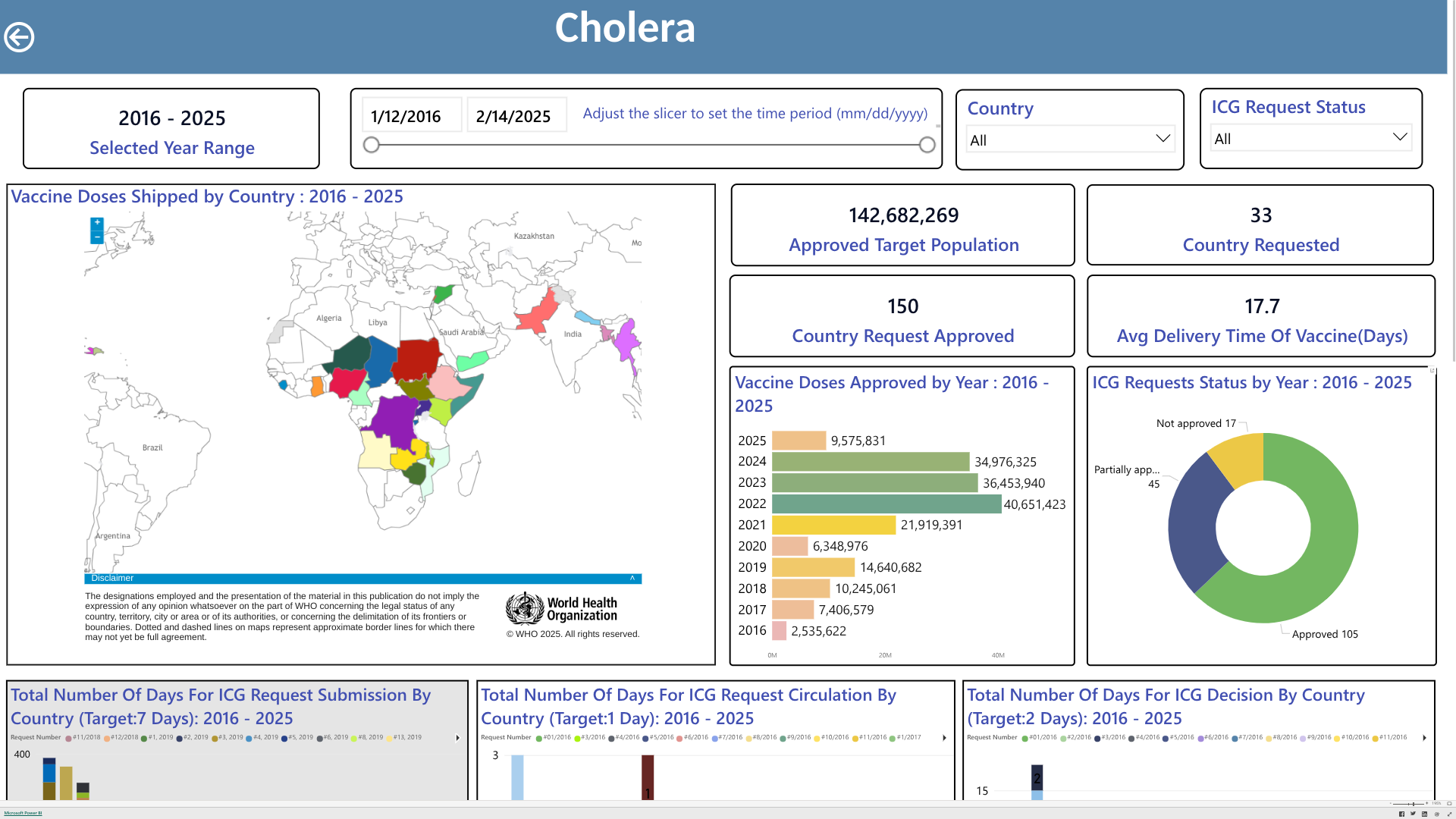
The World Health Organization (WHO) today announced two very positive trends related to the 7th cholera global outbreak.
On February 20, 2025, the WHO confirmed a 27% decrease in cholera cases since December 2024. A total of 34,799 new cholera and/or Acute Watery Diarrhoea cases were reported from 19 countries, territories, and areas across three WHO regions.
The period also saw a 33% decrease in related fatalities from the previous month.
This decrease was recorded despite a new cholera outbreak in Angola.
Additionally, the WHO reported that in January 2025, Oral Cholera Vaccine (OCV) production reached 6.2 million doses, a recent high point compared to December 2024, when 5.5 million doses were produced.
This progress follows introducing and prequalifying a new vaccine formulation and manufacturing process earlier in 2024.
However, the current OCV production has yet to meet growing global demand, and demand continues to exceed supply, says the WHO.
In the United States, OCVs will be offered at travel clinics and pharmacies in 2025.
The U.S. CDC's Advisory Committee on Immunization Practices (ACIP) today announced it has postponed next week's vaccine review meeting. This ACIP meeting was scheduled from February 26 to 28, 2025.
The draft agenda had been posted in early February.
As of February 20, 2025, the CDC's website stated, 'The ACIP workgroups met as scheduled this month and will present at the upcoming ACIP meeting.'
The next regularly scheduled ACIP meeting is in late June 2025.
The ACIP holds about three annual meetings, which are open to the public. At these meetings, scientists review scientific data and vote on vaccine recommendations. The ACIP recommendations are then passed on to the CDC's Director, who makes the final decisions.