Search API
Merck announced today that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) recommended the approval of CAPVAXIVE™ (Pneumococcal 21-valent Conjugate Vaccine) for active immunization for the prevention of invasive disease and pneumonia caused by Streptococcus pneumoniae in individuals 18 years of age and older.
Pneumococcal disease is an infection caused by Streptococcus pneumoniae. There are about 100 types of pneumococcal bacteria, and they can affect adults differently than children.
“Invasive pneumococcal disease and pneumococcal pneumonia remain critical public health challenges worldwide,” said Dr. Paula Annunziato, senior vice president of infectious diseases and vaccines, Global Clinical Development, Merck Research Laboratories, in a press release on January 31, 2025.
The CHMP’s recommendation for marketing authorization in the European Union (EU), Iceland, Liechtenstein, and Norway will now be reviewed by the European Commission. A final decision is expected by the second quarter of 2025.
If approved in the EU, it would mark the fourth authorization of CAPVAXIVE for preventing invasive pneumococcal disease and pneumococcal pneumonia in adults.
CAPVAXIVE was first approved in the U.S. in June 2024, Canada in July 2024, and Australia in January 2025. It is being reviewed in Japan, and other worldwide regulatory filings are underway.
In the U.S., pneumococcal vaccines are recommended for most people and are available at most community pharmacies. These vaccines may not work for everyone.
During 2024, the United States reported several measles outbreaks primarily related to unvaccinated international travelers. According to new reports, the State of Texas may lead this unfortunate list in 2025.
Today, the Texas Department of State Health Services (DSHS) announced two confirmed measles cases in Gaines County residents, located southwest of Lubbock, Texas. Both instances involve unvaccinated school-age children who were hospitalized in Lubbock.
As of January 30, 2025, these children have been discharged.
These newly identified cases are in addition to two confirmed measles cases reported in Harris County in 2025.
The Houston Health Department (HHD) identified two confirmed measles cases associated with international travel. Both adults were unvaccinated against measles.
HDD says anyone exposed to measles should monitor themselves for symptoms, including a rash, high fever, cough, runny nose, and red, watery eyes. Symptoms can appear 7 to 21 days after exposure. If you show symptoms of measles, call your healthcare provider to make arrangements for evaluation and treatment.
On January 23, 2025, HHD stated, 'Due to the highly contagious nature of this disease, additional (measles) cases may occur.'
Houston and Harris County are home to about 5 million people and are gateway cities with two international airports.
Crockett Tidwell RPh, CDCES, CTH, informed Vax-Before-Travel News, "Measles is extremely contagious; nine9 out 10 people in the same room will become infected if they do not have immunity."
"All it takes is one international traveler to infect every vulnerable person they come in contact with when they come home," added Tidwell, Clinical Services Manager, International Society of Travel Medicine Certificate in Travel Health™.
To alert travelers of the global measles risk, the U.S. CDC recently updated a Travel Health Advisory, which identified 59 countries reported measles cases. The CDC recommends people speak with a travel vaccine expert about immunization options before visiting these countries in 2025.

Takeda today announced earnings results for the third quarter of fiscal year 2024 (nine months ending December 31, 2024), showing continued advancement and demand for its dengue virus vaccine, Qdenga®.
The Company reported Qdenga's FY2024 H1 revenue was JPY 19.9B, reflecting 863% growth.
As of January 30, 2025, Qdenga is available in 27 countries, including 19 European countries, with travel recommendations to support using Qdenga to help protect travelers to dengue endemic areas.
For example, over the past year, dengue outbreaks have set new records in countries throughout the Region of the Americas.
In 2024, cities in the United States reported local dengue infections, including Los Angeles, California, and Miami, Florida.
Previously, the World Health Organization added Qdenga to its List of Prequalified Vaccines, which should expand the number of countries offering this second-generation dengue vaccine.
Unfortunately, Qdenga is not available in the United States.
On July 11, 2023, Takeda voluntarily withdrew the Biologics License Application following discussions with the U.S. Food and Drug Administration. As of 2025, there has been no indication approval discussions were pending.
Note: This VBT news article was update don January 5, 2025, to include current country authorizations.

Ocean Biomedical recently announced that its Scientific Co-founder, Dr. Jonathan Kurtis, MD, PhD, and his research team have received additional funding from the U.S. National Institutes of Health (NIH) to advance their malaria vaccine research.
With the support of a $4.6 million non-governmental Foundation grant, Dr. Kurtis’ team is now testing three vaccine candidates in non-human primates. These candidates aim to block the malaria parasite’s ability to enter and exit red blood cells.
The research also explores the feasibility of using lipid-encapsulated messenger ribonucleic acid (mRNA) technology as a delivery mechanism.
In December 2024, Dr. Kurtis secured a $3.5 million NIH grant to identify vaccine targets further to protect against severe malaria in children.
Malaria remains a devastating global health challenge, claiming the lives of over 500,000 children annually in sub-Saharan Africa.
As of January 30, 2025, two malaria vaccines are available in Africa. However, they are not available in the U.S.
Following the confirmation of an outbreak of Sudan virus disease in the Republic of Uganda, the World Health Organization (WHO) announced it is mobilizing efforts to support the national health authorities in containing a potential outbreak in Kampala.
The identification of the case in a densely populated urban requires a rapid and intense response, says the WHO.
As of January 30, 2025, a nurse from Mulago National Referral Hospital in the capital, Kampala, a city with about 1.8 million residents, has been reported with this disease.
A total of 45 contacts, including health workers and family members of the confirmed case (deceased), have been identified and are currently under close monitoring. No other health workers or patients have shown symptoms of the disease.
“We welcome the prompt declaration of this outbreak, and as a comprehensive response is being established, we are supporting the government and partners to scale up measures to quickly identify cases, isolate and provide care, curb the spread of the virus, and protect the population,” said Dr Matshidiso Moeti, WHO Regional Director for Africa, in a press release.
Eight previous outbreaks of the Sudan virus disease have occurred, five in Uganda and three in Sudan. Uganda last reported an outbreak in 2022.
Sudan virus disease is a severe, often fatal illness affecting humans and other primates. It is caused by Orthoebolavirus Sudanese (Sudan virus), a viral species belonging to the same genus as the virus that causes Ebola virus disease.
Case fatality rates of Sudan virus disease have varied from 41% to 100% in past outbreaks.
While no licensed vaccines for the Sudan virus disease exist, the WHO coordinates with developers to deploy candidate vaccines and other public health measures.
The WHO stated that experimental vaccines would be deployed once all administrative and regulatory approvals were obtained.
Since the Zika virus was first recognized in Africa in 1947, it has been detected globally in 92 countries and territories. With its first case in 2016, India has been an unfortunate leader in Zika cases.
Last year, a total of 151 Zika virus disease (ZVD) cases were reported from three states in India: Gujarat, Karnataka, and Maharashtra states.
Maharashtra reported a cumulative total of 140 ZVD cases in 2024, the highest since 2021.
Located in India's western region, Mumbai is Maharashtra's capital, with about 13 million residents.
As of January 29, 2025, the World Health Organization News reported that, based on current ZVD information, no travel or trade restriction with India is recommended.
However, the U.S. CDC issued a Level 2 - Practice Enhanced Precautions regarding Maharashtra's Zika outbreak in August 2024.
Last year, in the United States, the CDC reported 19 non-congenital Zika cases in U.S. residents (1 imported case in Texas).
The CDC says Zika is transmitted to humans by the bite of infected mosquitoes found throughout Puerto Rico.
Additionally, Zika is also transmitted from mother to fetus during pregnancy, as well as through sexual contact, transfusion of blood and blood products, and possibly through organ transplantation.
A recent study concluded young children diagnosed with congenital Zika syndrome had a 13-fold higher risk of morbidity compared with those without.
There is no specific treatment available for Zika virus infection or disease.
Furthermore, Zika vaccines have not yet been approved.
As vacationers plan their winter 2025 holiday trip to Florida's beautiful beaches to enjoy the warm weather, taking steps to prevent mosquito bites remains essential. In Florida, 14 species of Anopheles mosquitoes have been found to transmit diseases to humans.
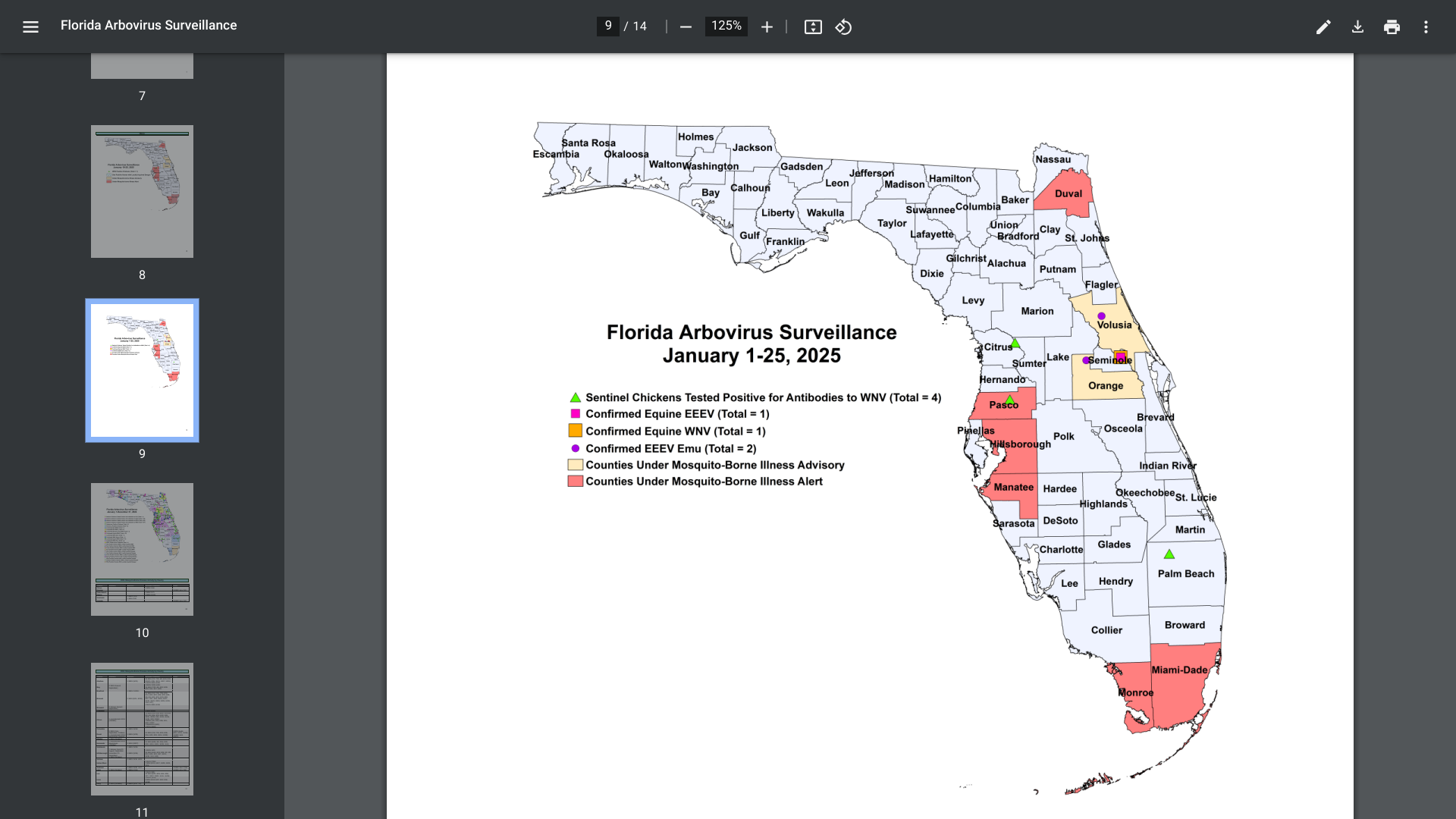
As of early January 2025, mosquito-borne diseases, such as dengue fever, have been reported again in Florida.
The Florida Department of Health (FDH) updated its Vaccine-Preventable Diseases Surveillance Report on January 25, 2025, confirming twenty-two cases of dengue were reported among persons who had international travel, and one locally acquired dengue case was reported.
Last year, 999 travel-associated dengue cases were reported, mainly among Brazil, Cuba (567), and Puerto Rico visitors.
Furthermore, 91 locally acquired dengue cases were reported from ten counties in 2024, led by Miami-Dade (50).
While dengue preventive vaccines remain unavailable in the United States, FDH and the U.S. CDC encourage all visitors to dengue-endemic areas to avoid mosquito bites. This CDC advice is particularly relevant for pregnant women, as the dengue virus has been detected in unborn infants.
The World Health Organization published its 46th situation report for the multi-country outbreak of monkeypox virus, including reports of new travel-related mpox cases due to clade Ib MPXV.
As of January 28, 2025, the WHO confirmed new travel-related clade Ib MPXV cases had been detected in countries that had already detected travel-related cases, including China, Germany, Thailand, Great Britain and Northern Ireland, and the United States. Azerbaijan has reported its first case during this outbreak.
The outbreak of clade Ib continues predominantly in the Democratic Republic of the Congo, Burundi, and Uganda.
Outside Africa, 11 countries have detected clade Ib MPXV.
In the U. S., since May 2022, most reported mpox cases are clade II.
Additionally, mpox vaccinations are commercially available at clinics and pharmacies in the U.S.

The UK Health Security Agency (UKHSA) today announced a confirmed case of influenza A(H5N1) in a person in the West Midlands region of Enhland.
The person acquired the infection on a farm, where they had close and prolonged contact with many infected birds. The birds were infected with the DI.2 genotype, one of the viruses circulating in birds in the UK this season.
As of January 27, 2025, the individual was admitted to a High Consequence Infectious Disease unit.
Despite extensive recent surveillance, no human-to-human transmission has been demonstrated. Therefore, the UKHSA has been tracing all individuals in contact with the confirmed case of avian influenza.
The first confirmed human case of A(H5N1) in England was in January 2022.
The UKHSA says the risk to the broader public remains very low in late January 2025.
In a press release, Minister for Public Health and Prevention Andrew Gwynne commented, "We recently added the H5 vaccine, which protects (people) against avian influenza, to our stockpile as part of our preparedness plans."
This person was infected with a different H5N1 strain circulating among mammals and birds in the United States.
The U.S. government has invested in avian influenza vaccines for people in the past few years.