Global Cholera Vaccine Stockpile is Depleted
According to the World Health Organization (WHO), the global Oral Cholera Vaccine (OCV) stockpile is depleted, and no doses are available.
The WHO's External Situation Report #19 says this shortage poses significant challenges to Cholera outbreak response efforts for the rest of 2024 and early 2025.
This news is concerning since around 1.3 billion people are at risk of cholera worldwide.
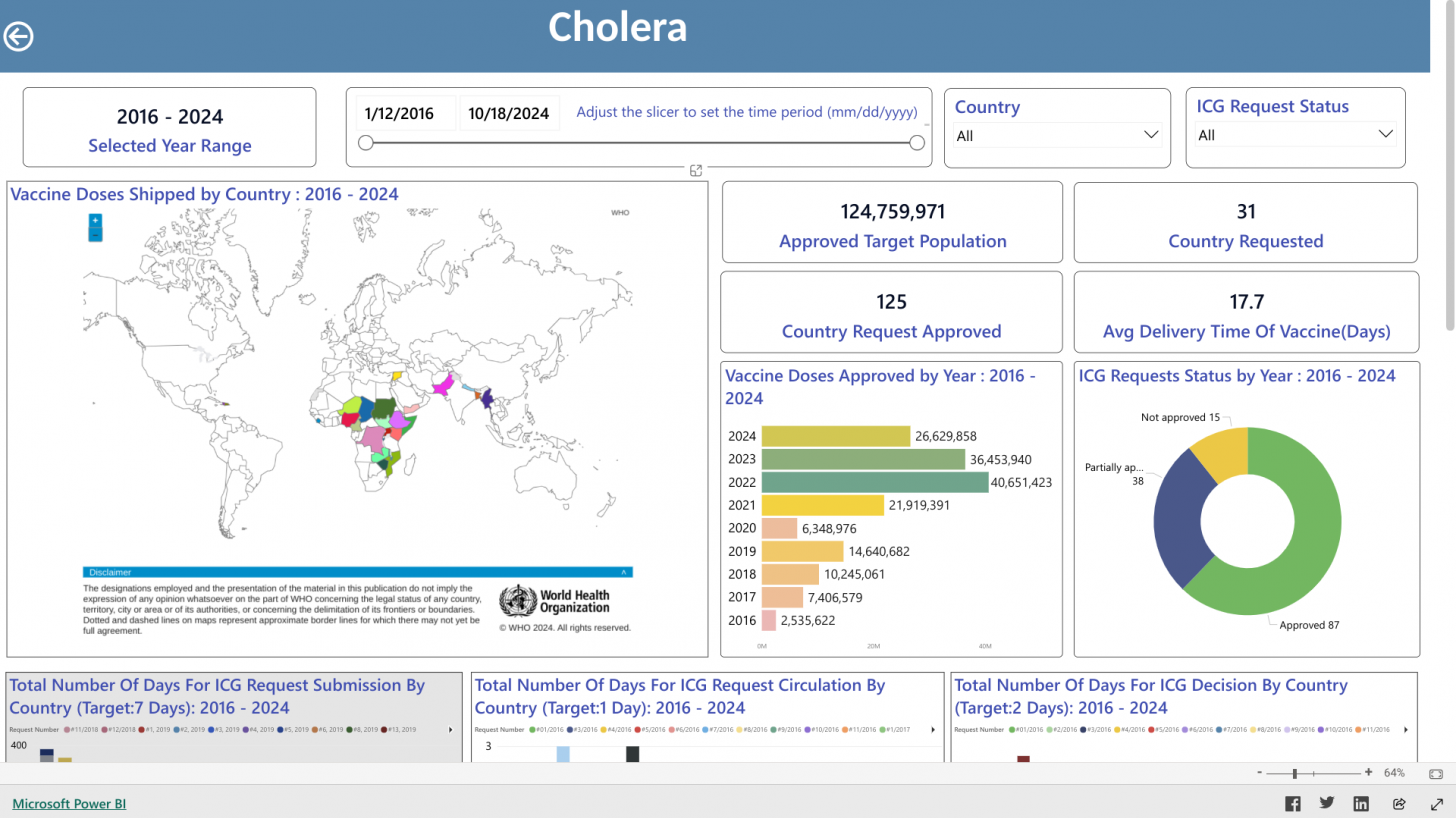
The International Coordinating Group (ICG) for OCVs was created in 1997 to manage the global stockpile. Since its establishment, the WHO, UNICEF, and Médecins sans Frontières have facilitated about 73 million doses of OCV for 23 countries.
As of October 14, 2024, the ICG dashboard indicates the global production capacity for this year is 37-50 million doses, with about 26 million approved for distribution.
Over the past month, the ICG received six requests for OCV from five countries: Bangladesh, Sudan, Niger, Ethiopia, and Myanmar. Due to limited vaccine availability, only 7.6 million doses could be shipped to these countries.
Recently, the Republic of Nigeria received about 900,000 OCV doses.
The WHO wrote on October 18, 2024, 'The shortfall in supply highlights the ongoing challenges in meeting global demand, especially as cholera outbreaks continue to rise across multiple regions. Efforts to scale production are underway, but immediate vaccine access remains constrained.'
"According to a Global Task Force on Cholera Control, the true impact of cholera is underestimated. Although global reports indicate between 100,000 and 1.2 million cases annually in the past two decades, cholera's prevalence is likely much higher due to gaps in surveillance, social stigma, and concerns about economic repercussions," said Duellyn Pandis, DNP, APRN, FNP-C.
"Currently, the changing climate elevates the risk of its spread to new regions," added Pandis, President & CEO of Passport Health of Tampa Bay.
OCVs such as Dukoral and Euvichol-Plus/Euvichol have been approved by the WHO, but they are unavailable in the U.S.
Cholera is spread through water and food contaminated with cholera bacteria. It can cause life-threatening watery diarrhea and vomiting.
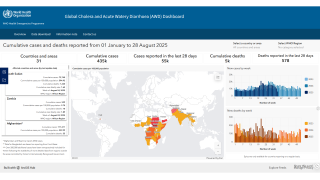
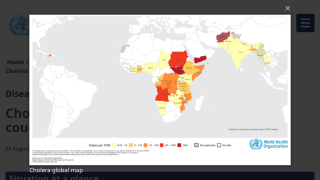
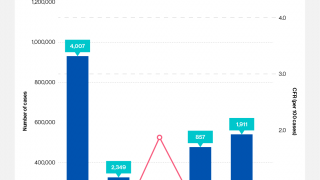
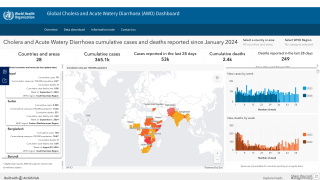
During 2024, a cumulative total of 439,724 cholera cases and 3,432 deaths were reported globally across five WHO regions. The 126% spike in deaths is deeply concerning, says the WHO.
In the United States, the single-dose Vaxchora is the only OCV approved for use by people ages 2 to 64 who are traveling to an area where cholera is present.
The U.S. CDC recommends future travelers to cholera-endemic areas speak with a vaccine expert about immunization options at least ten days before traveling abroad.
As of late October 2024, travel vaccine clinics and pharmacies in the U.S. generally have OCVs available.
Note: This article was updated in December 2024 to correct a typo.
Our Trust Standards: Medical Advisory Committee