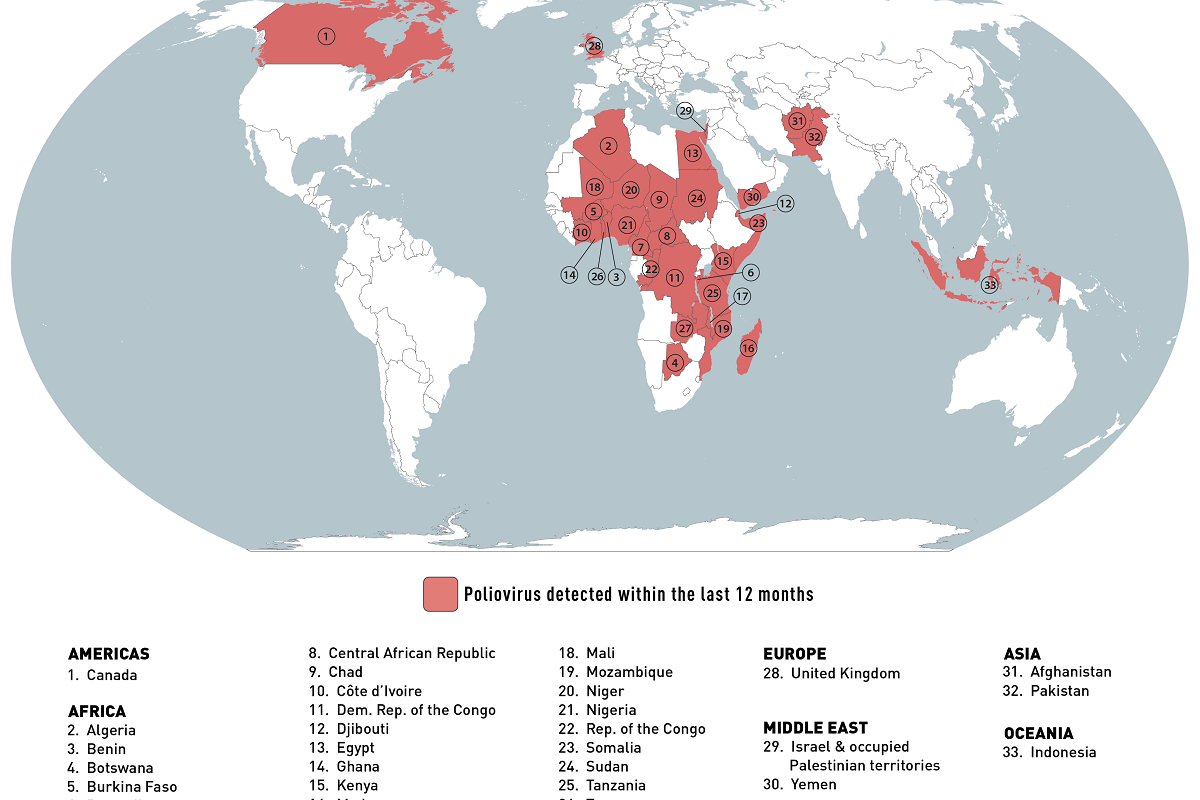
The World Health Organization (WHO) recently reported circulating vaccine-derived poliovirus type 2 (cVDPV2) cases in Africa.
The WHO confirmed on July 28, 2023, the United Republic of Tanzania reported the country's first cVDPV2 case and Kenya its first of 2023.
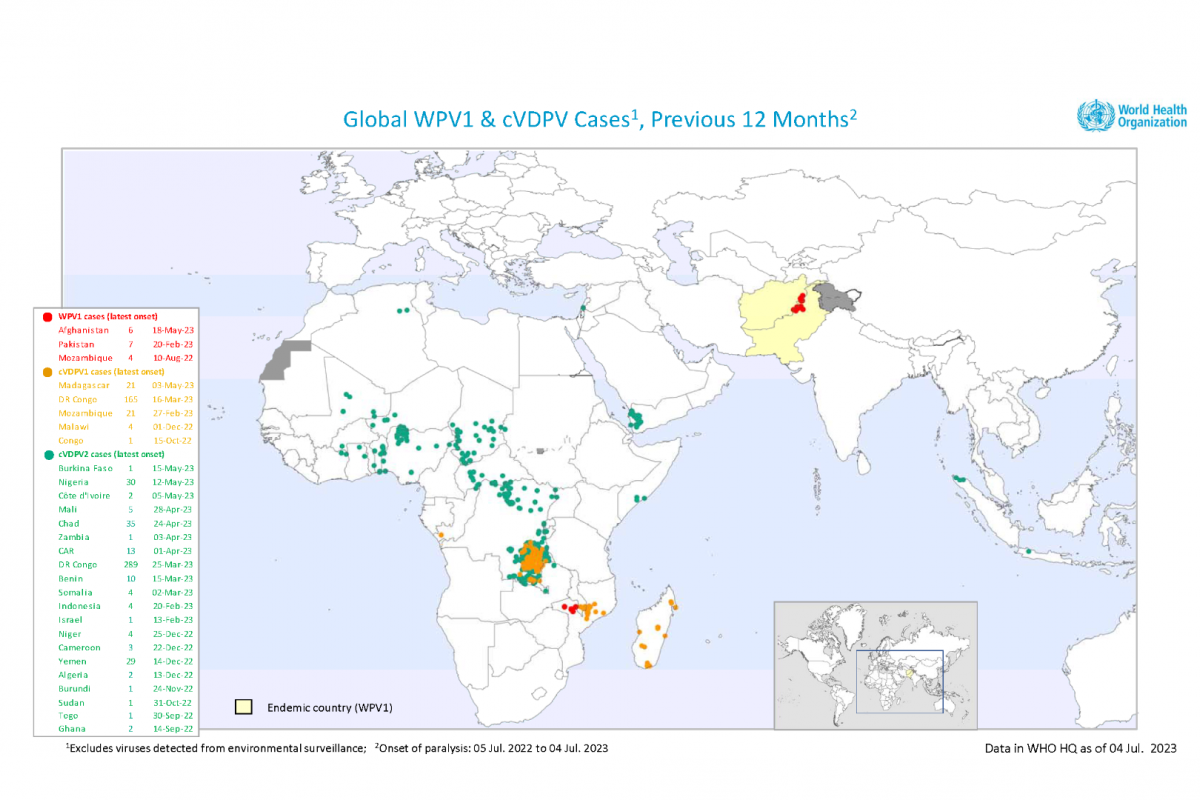
Tanzania's Ministry of Health notified the WHO the cVDPV2 virus was isolated from a case of acute flaccid paralysis (AFP) in the Rukwa region. Gene sequencing of the isolated virus has indicated close linkage with cVDPV2 currently circulating in South Kivu, Demographic Republic of the Congo.
According to the WHO-UNICEF estimates of national immunization coverage, the oral polio vaccine third dose (OPV3) and the inactivated polio vaccine first dose (IPV1) was 88% in Tanzania last year.
And on July 11, 2023, the WHO received an official report regarding detecting a cVDPV2 in two AFP cases and two asymptomatic healthy children community contacts in Kenya.
The genetic sequencing analyses showed that all four isolates are genetically linked to the cVDPV2 circulating in Banadir, Somalia.
Vaccine-derived poliovirus is a well-documented strain mutated from the strain originally contained in OPV.
OPV contains a live, weakened form of poliovirus that replicates in the intestine for a limited period, thereby developing immunity by building antibodies. On rare occasions, when replicating in the gastrointestinal tract, OPV strains genetically change and may spread in communities that are not fully vaccinated against polio, especially in areas with poor hygiene or overcrowding.
The lower the population's immunity, the longer this virus survives and the more genetic changes it undergoes.
In sporadic instances, the vaccine-derived virus can genetically change into a form that can cause paralysis, as does the wild poliovirus – this is what is known as a vaccine-derived poliovirus (VDPV).
The detection of VDPV in at least two different sources and at least two months apart that are genetically linked, showing evidence of transmission in the community is classified as cVDPV2.
In both countries, the WHO assesses the overall risk at the national level to be high due to the sub-optimal surveillance performance in some districts, sub-optimal vaccination coverage resulting in low population immunity, and the ongoing population movement across neighboring countries.
To alert international travelers, the U.S. CDC reissued its Level 2 - Practice Enhanced Precautions, Travel Health Advisory regarding the global polio outbreak on July 28, 2023.
The CDC says before any international travel, make sure you are up to date on your polio vaccines, and adults who previously completed the full, routine polio vaccine series may receive a single, lifetime booster dose of polio vaccine.
The nOPV2 vaccine is offered in Africa in July 2023. Approximately 670 million doses have been administered in more than 31 countries worldwide.
In the U.S., various polio vaccines are available at health clinics and community pharmacies.